Picture this: You’re scrolling through social media after an intense workout, your muscles still burning from that final set of burpees. Your phone battery is at 15% and desperately needs charging. What do you and your phone have in common? You both need energy to function, and you both have sophisticated systems for converting stored energy into usable power. Welcome to the fascinating world of Cellular Energetics – where biology meets chemistry to keep life running.
As an AP Biology student, you’re about to dive into one of the most fundamental and exam-heavy units in the entire course. Unit 3: Cellular Energetics accounts for 12-16% of your AP exam, making it crucial for your success. But here’s the good news: once you understand how energy flows through living systems, everything else in biology starts making perfect sense.
What You’ll Master in This Unit
By the end of this comprehensive guide, you’ll be able to:
- Explain how enzymes catalyze biological reactions and the factors that affect enzyme activity
- Describe the role of energy coupling in cellular processes
- Analyze the processes of cellular respiration and photosynthesis
- Compare and contrast different metabolic pathways
- Predict how changes in environmental conditions affect cellular energy processes
- Apply knowledge of cellular energetics to solve complex biological problems
Quick Check Knowledge Box #1:
Before we dive deeper, can you explain why a car engine and a muscle cell both need fuel? If you can connect these concepts, you’re already thinking like a biologist!
Chapter 1: The Energy Fundamentals – Why Cells Need Power
Understanding Energy in Biological Systems
Energy is the currency of life, but unlike the money in your wallet, biological energy comes in specific forms that cells can actually use. Think of adenosine triphosphate (ATP) as the universal credit card of cellular processes – accepted everywhere, from muscle contractions to protein synthesis.
But here’s where it gets interesting: cells can’t just create energy from nothing. They follow the same physical laws that govern everything else in the universe, particularly the laws of thermodynamics. The first law tells us that energy cannot be created or destroyed, only transformed. The second law reveals that every energy transformation results in some energy being lost as heat – which explains why you get warm during exercise.
Real-World Connection:
Consider your smartphone’s battery life. Just like your phone converts stored chemical energy into electrical energy to power apps, your cells convert glucose into ATP to power biological processes. And just like your phone generates heat during heavy use, your cells release heat during metabolism – that’s why you feel warm when you’re sick and your metabolism increases.
The ATP-ADP Cycle: Cellular Energy Currency
ATP (adenosine triphosphate) isn’t just another molecule – it’s the energetic heartbeat of every living cell. Picture ATP as a loaded spring: when the bond between the second and third phosphate groups breaks, energy is released to power cellular work. This leaves behind ADP (adenosine diphosphate), which is like an unloaded spring waiting to be compressed again.
The beauty of this system lies in its recyclability. Cells constantly regenerate ATP from ADP using energy from food molecules, creating an endless cycle that powers life. An average human recycles their body weight in ATP every single day – that’s roughly 250 kilograms of ATP production and breakdown!
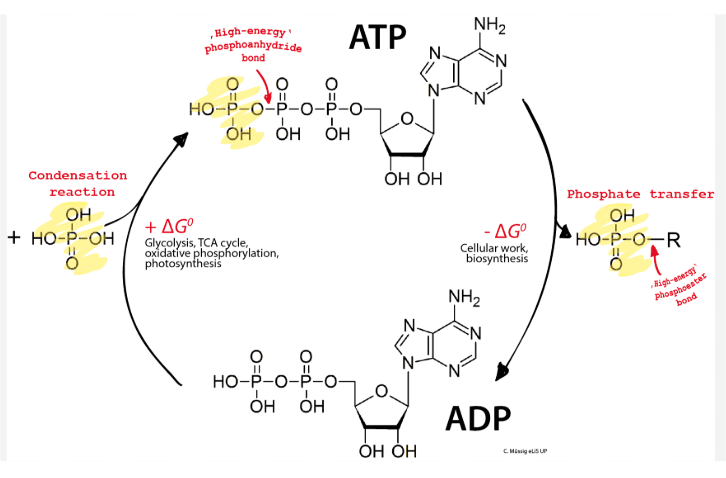
Study Tip:
To remember ATP’s structure, use the acronym “A-T-P”: Adenine base, ribose sugar (T for “three carbons visible”), and three Phosphate groups. The energy is stored in the phosphate bonds, specifically the terminal phosphate bond.
Energy Coupling: How Cells Get Work Done
Here’s a concept that trips up many students: cells don’t waste energy. Instead, they use energy coupling to link energy-releasing reactions (exergonic) with energy-requiring reactions (endergonic). It’s like using the energy from rolling a boulder downhill to push another boulder up a different hill.
Consider muscle contraction. The exergonic reaction of ATP hydrolysis (breaking ATP apart) provides energy for the endergonic process of myosin binding to actin. Without this coupling, muscle contraction would be impossible – your muscles would be as useful as a car without fuel.
Common Mistake Alert:
Students often think that ATP directly provides energy for all cellular processes. In reality, ATP provides energy through energy coupling. The energy released from ATP hydrolysis drives unfavorable reactions, making them thermodynamically possible.
Chapter 2: Enzymes – The Molecular Machines That Make Life Possible
How Enzymes Work: Lowering Activation Energy
Imagine trying to push a heavy box over a tall hill versus pushing it over a small bump. Enzymes essentially create that small bump pathway for chemical reactions. They don’t change the starting or ending point of a reaction – they just make it easier to get there by lowering the activation energy barrier.
Every enzyme has an active site – a specifically shaped region that binds to substrate molecules like a lock and key. But here’s the sophisticated part: the enzyme doesn’t just hold the substrate; it stabilizes the transition state, making the reaction proceed thousands or millions of times faster than it would naturally.
Real-World Connection:
Think about a professional matchmaker versus trying to find a relationship through random encounters. The matchmaker (enzyme) doesn’t change who you are or who you’re compatible with, but they dramatically increase the chances and speed of finding the right match (product formation).
Factors Affecting Enzyme Activity
Understanding enzyme kinetics isn’t just academic – it’s crucial for predicting how organisms respond to environmental changes. Let’s break down the key factors:
Temperature Effects:
As temperature increases, molecular motion increases, leading to more frequent enzyme-substrate collisions and faster reaction rates. However, excessive heat denatures enzymes by disrupting their three-dimensional structure. This explains why fever can be dangerous – elevated body temperature can impair essential enzymatic processes.
pH Effects:
Each enzyme has an optimal pH range where it functions best. Pepsin, which works in your highly acidic stomach, has an optimal pH around 2. In contrast, enzymes in your small intestines work best at pH 8-9. Changes in pH can alter the enzyme’s shape and charge distribution, affecting substrate binding and catalytic activity.
Substrate Concentration:
At low substrate concentrations, reaction rate increases proportionally with substrate concentration. However, at high concentrations, enzymes become saturated, and the reaction rate plateaus. This concept is crucial for understanding metabolic regulation.
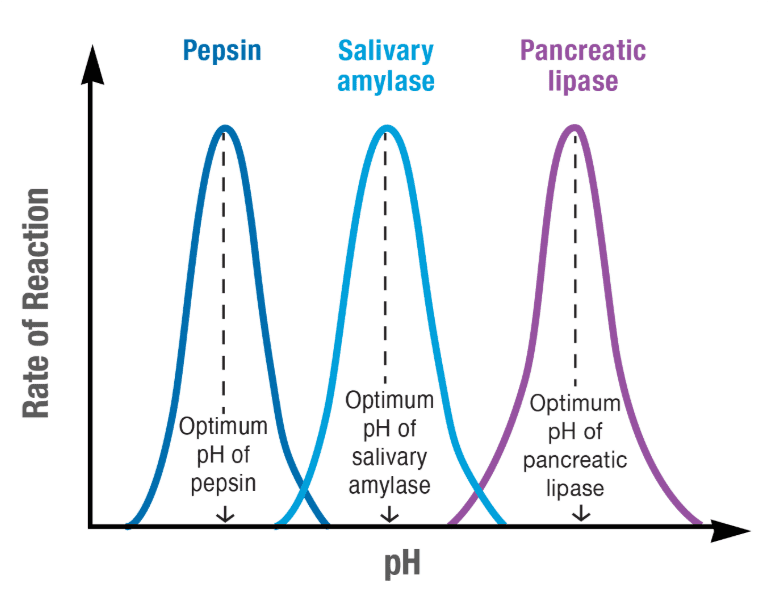
Quick Check Knowledge Box #2:
If you placed human enzymes in a test tube at 100°C, what would happen and why? Understanding this concept is key to predicting how extreme environments affect living organisms.
Enzyme Inhibition: Cellular Control Mechanisms
Cells need ways to control enzyme activity, and inhibition provides sophisticated regulatory mechanisms. Competitive inhibitors compete with substrates for the active site – like two people trying to sit in the same seat. Non-competitive inhibitors bind to a different site and change the enzyme’s shape, making it less effective – like someone adjusting your chair while you’re trying to work.
Feedback inhibition is particularly elegant: the end product of a metabolic pathway inhibits the first enzyme in the pathway. It’s like having a thermostat that turns off the heater when the room reaches the desired temperature. This prevents cells from wasting resources by overproducing unnecessary molecules.
Study Tip:
Create mental analogies for enzyme inhibition types. Competitive inhibition = musical chairs (competing for the same seat). Non-competitive inhibition = someone tying your shoelaces together (you can still walk, but not as effectively). Allosteric regulation = having multiple controls on a car (steering wheel, brakes, gas pedal all affect driving).
Chapter 3: Cellular Respiration – Extracting Energy from Food
Overview: From Glucose to ATP
Cellular respiration is essentially controlled combustion – your cells carefully extract energy from glucose molecules without creating the explosive heat you’d get from burning sugar with a match. This process occurs in three main stages: glycolysis, the Krebs cycle (citric acid cycle), and the electron transport chain.
The overall equation looks deceptively simple:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + ATP + heat
But this equation represents one of the most sophisticated energy conversion processes in nature, involving over 30 separate enzymatic reactions coordinated across different cellular compartments.
Real-World Connection:
Think of cellular respiration like a hydroelectric dam system. Water (electrons) flows from high energy (glucose) to low energy (oxygen), and this flow turns turbines (ATP synthase) to generate electricity (ATP). The dam doesn’t create energy – it captures and converts the energy of flowing water.
Glycolysis: The Universal Energy Pathway
Glycolysis occurs in the cytoplasm and doesn’t require oxygen, making it the most ancient and universal metabolic pathway. Every living organism, from bacteria to humans, can perform glycolysis. This 10-step process breaks one glucose molecule into two pyruvate molecules, producing a net gain of 2 ATP and 2 NADH.
Here’s what makes glycolysis fascinating: it’s both an energy-consuming and energy-producing process. The first five steps require an investment of 2 ATP molecules to activate glucose, but the last five steps produce 4 ATP molecules, resulting in a net profit of 2 ATP.
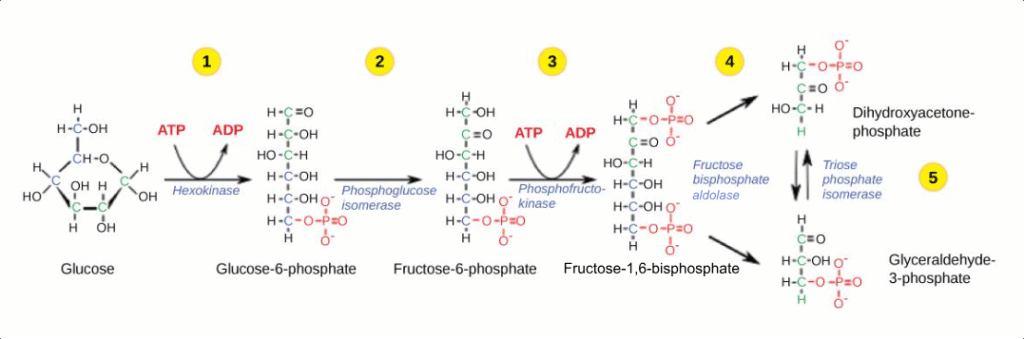
Study Tip:
Remember glycolysis produces: “2 ATP, 2 NADH, 2 pyruvate” – the “2-2-2 rule.” This helps you track energy yields throughout cellular respiration.
The Krebs Cycle: The Central Hub of Metabolism
Also called the citric acid cycle, this process occurs in the mitochondrial matrix and represents the complete oxidation of organic molecules. Each turn of the cycle processes one acetyl-CoA molecule (derived from pyruvate) and produces 3 NADH, 1 FADH₂, 1 ATP, and 2 CO₂.
What makes the Krebs cycle remarkable is its role as a metabolic hub. It’s not just for glucose breakdown – amino acids and fatty acids can also enter the cycle at various points. This integration explains how your body can convert proteins and fats into energy when carbohydrates are unavailable.
Common Mistake Alert:
Students often forget that glucose produces two pyruvate molecules, so the Krebs cycle runs twice per glucose molecule. Don’t forget to double your Krebs cycle products when calculating total ATP yield from glucose!
Electron Transport Chain: The Power Plant of the Cell
The electron transport chain (ETC) is where the majority of ATP production occurs. Located in the inner mitochondrial membrane, this system uses the NADH and FADH₂ produced in earlier stages to create a proton gradient that drives ATP synthesis.
Think of the ETC as a series of waterfalls. Electrons flow from high-energy carriers (NADH, FADH₂) through a series of protein complexes, losing energy at each step. This energy pumps protons across the inner mitochondrial membrane, creating a concentration gradient. ATP synthase harnesses this gradient, much like a water wheel harnesses flowing water.
The final electron acceptor is oxygen, which combines with protons to form water. This explains why oxygen is essential for complex life – without it, the electron transport chain stops, ATP production ceases, and cells die within minutes.
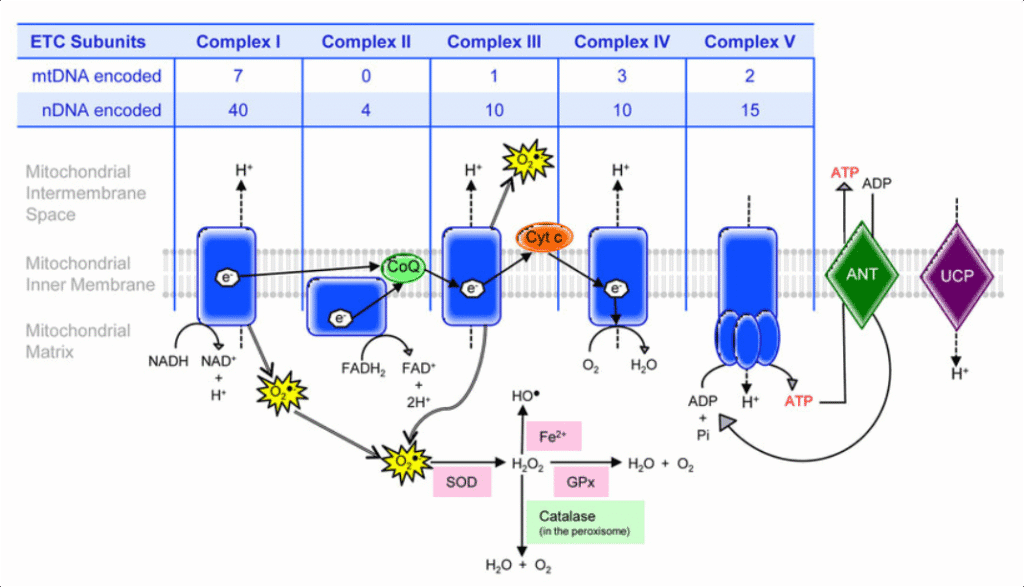
Quick Check Knowledge Box #3:
Why do we breathe oxygen, and what happens to it in our cells? If you can explain the role of oxygen as the final electron acceptor, you understand one of biology’s most crucial processes.
Fermentation: Life Without Oxygen
When oxygen isn’t available, cells can still extract some energy through fermentation. This process regenerates NAD⁺ from NADH, allowing glycolysis to continue. However, fermentation produces only 2 ATP per glucose molecule compared to the 30-32 ATP produced through complete cellular respiration.
Humans primarily use lactic acid fermentation during intense exercise when oxygen delivery to muscles is insufficient. The burning sensation in your muscles during a hard workout is partly due to lactic acid accumulation. Yeast uses alcoholic fermentation, converting pyruvate to ethanol and CO₂ – the basis for bread rising and alcohol production.
Real-World Connection:
Understanding fermentation explains why athletes train at high intensities. Regular exposure to oxygen-limited conditions improves the body’s ability to clear lactate and maintain performance during anaerobic exercise.
Chapter 4: Photosynthesis – Capturing Solar Energy
The Big Picture: Converting Light to Chemical Energy
Photosynthesis is arguably the most important biological process on Earth. It converts solar energy into chemical energy, produces the oxygen we breathe, and forms the base of virtually all food webs. The overall equation appears to be the reverse of cellular respiration:
6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂
However, this simple equation masks incredible complexity. Photosynthesis actually consists of two interconnected stages: light-dependent reactions (photo part) and light-independent reactions (synthesis part).
Real-World Connection:
Solar panels and photosynthesis both capture light energy, but photosynthesis is far more sophisticated. While the best solar panels convert about 20% of sunlight to electricity, photosynthesis converts light energy to chemical energy and simultaneously produces oxygen, removes CO₂ from the atmosphere, and creates the organic molecules that feed most life on Earth.
Light-Dependent Reactions: The Photo Phase
These reactions occur in the thylakoid membranes of chloroplasts and directly capture light energy. Chlorophyll and other pigments absorb photons, exciting electrons to higher energy levels. This process is similar to solar panels but with biological precision.
The light-dependent reactions accomplish three crucial tasks:
- Split water molecules, releasing oxygen as a byproduct
- Generate ATP through photophosphorylation
- Produce NADPH, an electron carrier used in the Calvin cycle
Photosystem II absorbs light energy and uses it to extract electrons from water, while Photosystem I further energizes these electrons to reduce NADP⁺ to NADPH. The electron transport chain between these photosystems pumps protons into the thylakoid space, creating a gradient that drives ATP synthesis.
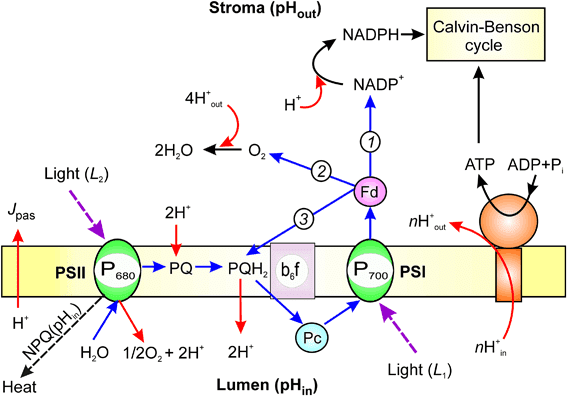
Study Tip:
Remember that Photosystem II comes before Photosystem I in the electron flow, even though it’s numbered higher. They were discovered and named in reverse order of their function. Think “PS-II then PS-I” in the sequence of electron flow.
The Calvin Cycle: Carbon Fixation
The Calvin cycle, also called light-independent reactions, uses the ATP and NADPH produced in the light-dependent reactions to convert CO₂ into organic molecules. This process occurs in the chloroplast stroma and doesn’t directly require light, though it depends on the products of light-dependent reactions.
The cycle has three phases:
- Carbon fixation: CO₂ combines with RuBP (ribulose bisphosphate) catalyzed by RuBisCO
- Reduction: 3-phosphoglycerate molecules are reduced using ATP and NADPH
- Regeneration: RuBP is regenerated to continue the cycle
For every three turns of the Calvin cycle, one G3P (glyceraldehyde 3-phosphate) molecule exits to eventually form glucose, while five G3P molecules regenerate three RuBP molecules.
Common Mistake Alert:
Students often think the Calvin cycle produces glucose directly. Actually, two G3P molecules combine to form one glucose molecule, requiring six turns of the Calvin cycle per glucose produced.
Factors Affecting Photosynthesis
Understanding how environmental factors affect photosynthesis helps predict plant responses to climate change and optimize agricultural practices.
Light Intensity: At low light levels, photosynthesis rate increases proportionally with light intensity. However, at high intensities, other factors become limiting, and the rate plateaus.
CO₂ Concentration: Since CO₂ is a raw material for photosynthesis, increased atmospheric CO₂ can enhance photosynthesis rates, but this effect is often limited by other factors.
Temperature: Higher temperatures generally increase reaction rates, but excessive heat can denature enzymes and damage chloroplast structure.
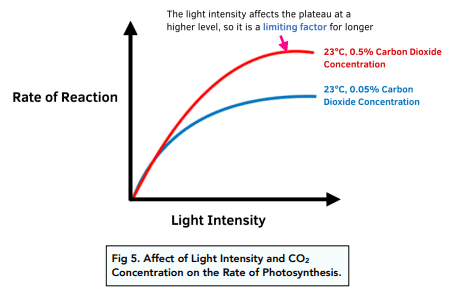
Quick Check Knowledge Box #4:
Why might a greenhouse use artificial lights, elevated CO₂, and controlled temperature? Understanding limiting factors in photosynthesis explains many agricultural practices.
Chapter 5: Metabolic Pathways and Regulation
Interconnected Metabolic Networks
Metabolism isn’t just about cellular respiration and photosynthesis – it’s a vast network of interconnected pathways that allow cells to build, break down, and modify molecules as needed. Anabolic pathways consume energy to build complex molecules, while catabolic pathways release energy by breaking down complex molecules.
This metabolic flexibility explains how your body can switch between using carbohydrates, fats, and proteins for energy depending on availability and need. During fasting, your liver converts stored glycogen to glucose. During extended fasting, it can even convert amino acids and fatty acids into glucose through gluconeogenesis.
Real-World Connection:
Think of metabolism like a city’s infrastructure. Just as a city has interconnected systems for transportation, power, water, and waste management, cells have interconnected metabolic pathways for energy production, building materials, waste removal, and communication. Disruption in one system affects the entire network.
Regulation of Metabolic Pathways
Cells must carefully regulate metabolic pathways to avoid waste and maintain homeostasis. This regulation occurs through several mechanisms:
Allosteric Regulation: Enzymes have binding sites separate from the active site where regulatory molecules can bind and change enzyme activity. This allows for rapid response to changing cellular conditions.
Covalent Modification: Adding or removing phosphate groups can quickly activate or deactivate enzymes. This is particularly important in regulating key enzymes in glycolysis and gluconeogeneus.
Enzyme Induction/Repression: Cells can increase or decrease enzyme production based on need. This provides longer-term regulation of metabolic capacity.
Compartmentalization: Different metabolic pathways occur in different cellular locations, allowing for independent regulation and preventing conflicting reactions.
Study Tip:
Use the acronym “ACEC” to remember regulatory mechanisms: Allosteric regulation, Covalent modification, Enzyme induction/repression, and Compartmentalization.
Metabolic Disorders and Their Implications
Understanding normal metabolism helps explain various metabolic disorders. Diabetes results from impaired glucose regulation, leading to elevated blood glucose levels and disrupted cellular energy metabolism. Phenylketonuria (PKU) involves a defective enzyme that cannot break down the amino acid phenylalanine, leading to toxic accumulations.
These disorders highlight the precision required for normal metabolic function and demonstrate how single enzyme defects can have system-wide consequences.
Common Mistake Alert:
Students sometimes think metabolic disorders only affect energy production. In reality, they can affect any aspect of metabolism, including synthesis of essential molecules, breakdown of toxic compounds, and regulation of metabolic flux.
Chapter 6: Energy Flow in Ecosystems
Connecting Cellular and Ecological Energetics
The energy transformations occurring in individual cells scale up to determine energy flow through entire ecosystems. Primary producers (plants and other photosynthetic organisms) capture solar energy and convert it to chemical energy. This energy then flows through food webs as organisms consume each other.
However, energy transfer between trophic levels is inefficient – typically only 10% of energy is transferred from one level to the next. This 10% rule explains why food webs rarely have more than four or five trophic levels and why there are fewer predators than prey organisms.
Real-World Connection:
The inefficiency of energy transfer explains why eating lower on the food chain is more environmentally sustainable. Producing one pound of beef requires about 16 pounds of grain because cattle must use most of the grain’s energy for their own metabolism, with only a small fraction converted to meat.
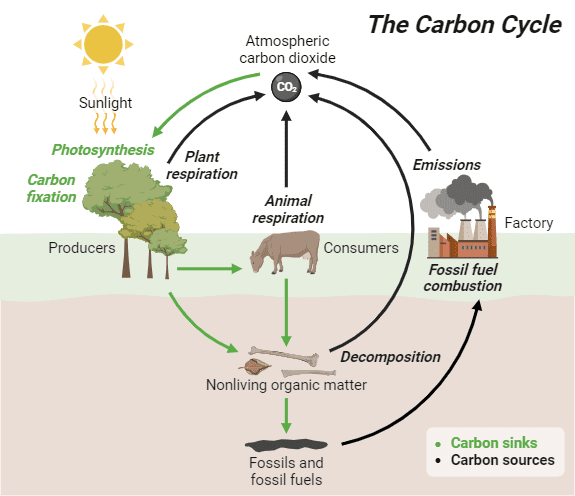
Biogeochemical Cycles and Energy
While energy flows through ecosystems in one direction (from sun to heat), nutrients cycle through ecosystems repeatedly. The carbon cycle links photosynthesis and cellular respiration on a global scale. CO₂ removed from the atmosphere during photosynthesis returns through cellular respiration, decomposition, and combustion.
Climate change represents a disruption of this balance, with human activities releasing CO₂ faster than photosynthesis can remove it from the atmosphere.
Quick Check Knowledge Box #5:
How do the processes you’ve learned about in individual cells contribute to global climate patterns? Making these connections demonstrates mastery of biological systems thinking.
Chapter 7: Advanced Topics and Current Research
Metabolic Engineering and Biotechnology
Scientists are now applying knowledge of cellular energetics to solve practical problems. Metabolic engineering involves modifying organisms’ metabolic pathways to produce useful compounds. For example, bacteria have been engineered to produce biofuels, pharmaceuticals, and industrial chemicals using modified versions of natural metabolic pathways.
CRISPR technology allows precise modification of genes encoding key enzymes, enabling researchers to optimize metabolic pathways for specific purposes. This field represents the practical application of fundamental knowledge about cellular energetics.
Evolutionary Perspectives on Metabolism
The metabolic pathways you’ve studied evolved over billions of years. Glycolysis likely evolved first, when Earth’s atmosphere lacked oxygen. Photosynthesis evolved later and dramatically changed Earth’s atmosphere by producing oxygen. Cellular respiration evolved to take advantage of oxygen’s high electron affinity.
Understanding this evolutionary history explains why glycolysis occurs in the cytoplasm (where it evolved in ancient prokaryotes) while the Krebs cycle and electron transport chain occur in mitochondria (which evolved from engulfed bacteria).
Real-World Connection:
The endosymbiotic theory explains why mitochondria have their own DNA and ribosomes – they were once independent bacteria that formed a symbiotic relationship with early eukaryotic cells. This partnership was so successful that it became permanent, creating the energy-efficient eukaryotic cells that allowed complex life to evolve.
Metabolic Flexibility and Adaptation
Different organisms have evolved various strategies for dealing with energy challenges. Some animals can enter torpor or hibernation to reduce energy needs during resource scarcity. Others, like camels, have metabolic adaptations that allow them to survive in harsh environments.
Plants have evolved different photosynthetic strategies (C3, C4, and CAM photosynthesis) to optimize carbon fixation under different environmental conditions. C4 and CAM plants have adaptations that minimize photorespiration, a wasteful process that competes with photosynthesis.
Study Tip:
Understanding metabolic adaptations helps predict how organisms might respond to environmental changes. This type of analysis question frequently appears on AP Biology exams.
Chapter 8: Laboratory Applications and Data Analysis
Enzyme Activity Labs
Understanding enzyme kinetics prepares you for laboratory investigations involving enzyme activity. These labs typically examine how factors like temperature, pH, substrate concentration, or inhibitors affect enzyme function.
When analyzing enzyme lab data, look for:
- Optimal conditions where enzyme activity is highest
- Points where enzyme activity begins to decline due to denaturation
- Evidence of competitive vs. non-competitive inhibition
- Relationship between substrate concentration and reaction rate
Real-World Connection:
Enzyme kinetics studies are crucial for drug development. Many medications work by inhibiting specific enzymes involved in disease processes. Understanding how inhibitors affect enzyme activity helps researchers design more effective treatments.
Photosynthesis and Cellular Respiration Labs
These investigations often measure gas exchange (O₂ and CO₂) to track photosynthesis and cellular respiration rates. Understanding the underlying processes helps you interpret data and design controlled experiments.
Key experimental considerations:
- Light vs. dark conditions for measuring net vs. gross photosynthesis
- Temperature effects on metabolic rates
- Impact of different wavelengths of light on photosynthesis
- Comparing photosynthesis rates in different plant species
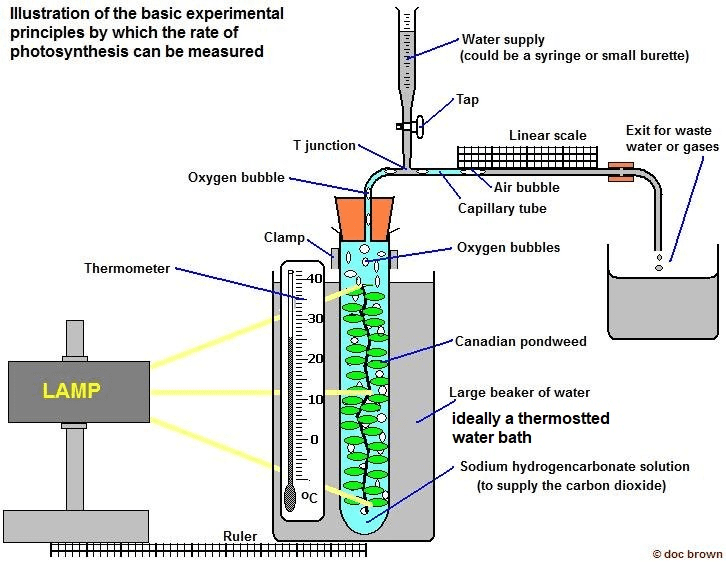
Quick Check Knowledge Box #6:
If you measured oxygen production by aquatic plants under red, blue, and green light, which would you expect to produce the most oxygen and why? This connects light absorption by chlorophyll to photosynthesis rates.
Data Interpretation Skills
AP Biology exams frequently include data interpretation questions related to cellular energetics. Practice analyzing:
- Graphs showing enzyme activity under different conditions
- Data tables comparing metabolic rates in different organisms
- Experimental results testing hypotheses about metabolic processes
- Scientific papers describing metabolic research
Study Tip:
When analyzing graphs, always identify the independent variable (x-axis), dependent variable (y-axis), and any trends or patterns. Consider what biological processes could explain the observed data patterns.
Practice Questions and Assessment
Multiple Choice Questions
Question 1: Which of the following best explains why ATP is considered the “universal energy currency” of cells?
A) ATP can be stored in large quantities for long periods
B) ATP contains more energy per molecule than any other compound
C) ATP can couple exergonic and endergonic reactions efficiently
D) ATP is the only molecule that can provide energy for cellular work
Answer: C. ATP’s role as energy currency comes from its ability to couple energy-releasing reactions with energy-requiring reactions, not from storing large amounts of energy or being the only energy source.
Question 2: A student measures oxygen consumption in germinating seeds at different temperatures. The rate increases from 0°C to 30°C, then decreases from 30°C to 50°C. This pattern most likely results from:
A) Increased substrate availability at higher temperatures
B) Enzyme denaturation at temperatures above 30°C
C) Decreased oxygen solubility at higher temperatures
D) Temperature-dependent changes in seed metabolism only
Answer: B. The initial increase reflects higher enzyme activity with temperature, but the decrease above 30°C indicates enzyme denaturation, which reduces metabolic efficiency.
Question 3: In C4 plants, the first product of carbon fixation is a 4-carbon compound. This adaptation primarily helps plants:
A) Increase the rate of the Calvin cycle
B) Reduce photorespiration in hot, dry conditions
C) Produce more ATP during photosynthesis
D) Absorb light energy more efficiently
Answer: B. C4 photosynthesis concentrates CO₂ around RuBisCO, reducing the enzyme’s tendency to bind oxygen instead of CO₂ (photorespiration), which is particularly problematic in hot, dry conditions.
Free Response Question Example
Question: An experiment was conducted to investigate the effect of pH on the activity of pepsin, an enzyme found in the stomach.
a) Describe the relationship between pH and pepsin activity shown in the data.
b) Explain why pepsin shows this pH-activity relationship in terms of enzyme structure and function.
c) Predict what would happen to pepsin activity if the enzyme were moved to the small intestine (pH ~8) and justify your prediction.
d) Design an experiment to test whether the pH effect on pepsin is reversible.
Sample Answer Framework:
a) Pepsin activity is highest at pH 2, decreases gradually from pH 3-5, and shows minimal activity at pH 6-8, creating an optimal pH range around 2.
b) Pepsin evolved to function in the acidic stomach environment. Its amino acid sequence creates a three-dimensional structure that is stable and properly shaped only at low pH. Higher pH values alter the enzyme’s charge distribution and shape, reducing substrate binding affinity and catalytic efficiency.
c) In the small intestine (pH 8), pepsin activity would be minimal because the high pH would denature the enzyme by disrupting its optimized structure. This makes biological sense because different enzymes (like trypsin and chymotrypsin) function in the alkaline small intestine.
d) To test reversibility, expose pepsin to high pH (pH 8) for a set time, then return it to optimal pH (pH 2) and measure activity recovery. Include controls: pepsin kept at pH 2 throughout, and pepsin kept at pH 8 throughout. If the effect is reversible, activity should return when pH is restored to 2.
Quick Check Knowledge Box #7:
Can you explain why your stomach produces hydrochloric acid beyond just “for digestion”? Understanding enzyme specificity and optimal conditions explains many physiological adaptations.
Data Analysis Practice
Scenario: A researcher studies photosynthesis rates in aquatic plants under different light intensities, measuring oxygen production as an indicator of photosynthetic activity.
Analysis Questions:
- Identify the light compensation point and explain its biological significance.
- Explain why oxygen production rate plateaus at high light intensities.
- Predict how this curve might change if CO₂ concentration were increased.
- What does the slope of the initial linear portion represent?
Study Tip:
Practice interpreting data from different types of experiments. The AP exam frequently includes novel experimental scenarios that test your ability to apply fundamental principles to new situations.
Common Mistakes and How to Avoid Them
Mistake #1: Confusing Energy Storage with Energy Transfer
Many students think ATP stores large amounts of energy for long periods. In reality, ATP is rapidly cycled – the same ATP molecule might be created and broken down hundreds of times per day. Cells store energy long-term in molecules like glycogen and fats, but use ATP for immediate energy transfer.
Mistake #2: Forgetting Cellular Respiration Produces Water
The equation for cellular respiration shows water as a product, but students often focus only on CO₂ production. Remember that oxygen combines with hydrogen ions and electrons at the end of the electron transport chain to form water. This is why cellular respiration is also called “internal respiration.”
Mistake #3: Thinking Photosynthesis Only Occurs During the Day
While light-dependent reactions require light, the Calvin cycle can continue in darkness as long as ATP and NADPH are available. Some plants (CAM plants) even fix CO₂ primarily at night to reduce water loss.
Mistake #4: Oversimplifying Enzyme Inhibition
Competitive inhibition can be overcome by increasing substrate concentration, but non-competitive inhibition cannot. Understanding this difference is crucial for predicting how cells regulate metabolic pathways.
Common Mistake Alert:
Don’t memorize ATP yields as exact numbers. Different sources give slightly different values (30-32 ATP per glucose for cellular respiration) because the actual yield depends on transport efficiency and other factors. Focus on understanding the processes rather than memorizing specific numbers.
Mistake #5: Ignoring Evolutionary Context
Modern metabolic pathways evolved over billions of years. Understanding this history explains seemingly inefficient aspects like photorespiration and helps predict how organisms might adapt to environmental changes.
Quick Check Knowledge Box #8:
Why might RuBisCO, the most abundant enzyme on Earth, be relatively inefficient at distinguishing CO₂ from O₂? Evolutionary context explains many biological “imperfections.”
Study Strategies and Exam Preparation
Creating Effective Study Materials
Concept Maps: Create visual representations showing how different metabolic pathways connect. Link glycolysis to fermentation and cellular respiration. Connect photosynthesis to the Calvin cycle and cellular respiration through CO₂ and O₂ exchange.
Process Diagrams: Draw detailed diagrams of key processes from memory, then check for accuracy. Include enzyme names, substrates, products, and energy changes for each step.
Comparison Tables: Create tables comparing aerobic vs. anaerobic respiration, different types of fermentation, C3 vs. C4 vs. CAM photosynthesis, and competitive vs. non-competitive inhibition.
Time Management for Unit 3
Week 1-2: Master enzyme structure, function, and kinetics. Practice interpreting enzyme activity graphs and understanding factors affecting enzyme function.
Week 3-4: Focus on cellular respiration pathways. Understand each stage’s location, inputs, outputs, and regulation. Practice calculating ATP yields and tracking carbon atoms through the pathways.
Week 5-6: Study photosynthesis in detail. Connect light-dependent and light-independent reactions. Understand how environmental factors affect photosynthetic efficiency.
Week 7: Review metabolic regulation and connections between pathways. Practice data interpretation and experimental design questions.
Week 8: Take practice tests and review weak areas. Focus on applying concepts to novel scenarios.
Exam-Specific Strategies
For Multiple Choice Questions:
- Read questions carefully – they often ask about specific aspects of processes
- Eliminate obviously incorrect answers first
- Use process of elimination when unsure
- Watch for questions testing common misconceptions
For Free Response Questions:
- Address all parts of the question
- Use specific biological terminology correctly
- Explain your reasoning, don’t just state facts
- Draw diagrams when they help clarify your explanation
- Connect molecular processes to larger biological principles
Study Tip: The “Teaching Test”
Explain cellular energetics concepts to someone else (friend, family member, or even an imaginary student). If you can teach it clearly, you understand it well enough for the AP exam.
Self-Assessment Checklist
Before moving on from Unit 3, ensure you can:
□ Explain how enzyme structure relates to function and specificity
□ Predict how changes in temperature, pH, and inhibitors affect enzyme activity
□ Trace the flow of energy and matter through cellular respiration pathways
□ Compare and contrast different metabolic strategies (aerobic, anaerobic, fermentation)
□ Explain how photosynthesis captures and converts light energy
□ Analyze data from experiments involving metabolic processes
□ Connect cellular processes to ecosystem-level energy flow
□ Predict how environmental changes affect metabolic efficiency
Real-World Connection:
Understanding cellular energetics prepares you for careers in medicine, biotechnology, environmental science, and agriculture. Doctors use this knowledge to understand metabolic disorders. Biotechnologists engineer metabolic pathways to produce useful compounds. Environmental scientists apply these principles to understand ecosystem responses to climate change.
Conclusion: Mastering the Energy of Life
Congratulations! You’ve journeyed through one of biology’s most fundamental and fascinating topics. From the molecular machines called enzymes to the global cycling of carbon, you’ve explored how energy flows through living systems at every scale.
Unit 3: Cellular Energetics isn’t just about memorizing pathways and equations – it’s about understanding the elegant solutions life has evolved to capture, convert, and utilize energy. These processes connect every living thing on Earth through shared biochemical heritage and ongoing ecological relationships.
As you prepare for the AP Biology exam, remember that this unit provides the foundation for understanding many other biological concepts. Cellular energetics explains why organisms have the forms they do, how they respond to environmental changes, and how life has evolved and diversified over billions of years.
Your Next Steps
- Review regularly: Cellular energetics concepts build on each other. Regular review prevents knowledge gaps from developing.
- Practice with real data: Work with authentic experimental data to develop data interpretation skills essential for AP success.
- Make connections: Link cellular processes to organismal biology, ecology, and evolution. The AP exam rewards systems thinking.
- Teach others: Explaining concepts to classmates reinforces your own understanding and reveals areas needing additional study.
- Stay curious: Follow current research in metabolism, bioenergetics, and related fields. Science is constantly advancing our understanding of these fundamental processes.
Final Quick Check Knowledge Box:
Can you explain how the morning cup of coffee in your kitchen connects to photosynthesis, cellular respiration, enzyme kinetics, and global carbon cycling? If so, you’ve truly mastered cellular energetics!
Remember, mastering cellular energetics takes time and practice, but the investment pays off not just on the AP exam, but in developing a deep appreciation for the remarkable chemistry of life. Every breath you take, every step you walk, and every thought you think depends on the processes you’ve studied in this unit.
The energy flowing through your cells right now as you read these words represents an unbroken chain of energy transformations stretching back billions of years to the first organisms that learned to harness the power of sunlight and chemical bonds. You are, quite literally, part of the grand energy story of life on Earth.
Good luck with your studies, and remember – understanding how life harnesses energy is understanding one of the most profound accomplishments of evolution. You’re not just learning for an exam; you’re discovering the fundamental processes that make your very existence possible.
Also Read –

