Why States of Matter Matter to You
Have you ever wondered why ice melts in your drink on a hot day, or why steam rises from your morning coffee? Welcome to the fascinating world of states of matter – one of the most fundamental topics in IGCSE Chemistry that explains everything from the water cycle to how your smartphone screen works!
As an IGCSE Chemistry student, mastering Topic 1: States of Matter isn’t just about passing your exam (though it definitely helps!). This topic forms the foundation for understanding almost everything else in chemistry. From chemical reactions to industrial processes, the behavior of particles in different states affects it all.
Don’t worry if you’re feeling overwhelmed – we’re going to break this down into bite-sized, digestible pieces that will have you confidently answering exam questions in no time. Let’s dive in and discover why this topic is actually much more exciting than you might think!
Understanding the Basics – What Are States of Matter?
The Three Main States You Need to Know
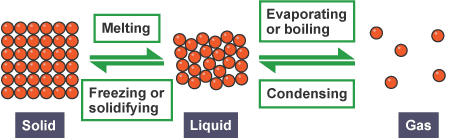
Matter exists in three primary states that you encounter every single day:
Solid State:
- Think of ice cubes in your freezer or the desk you’re sitting at
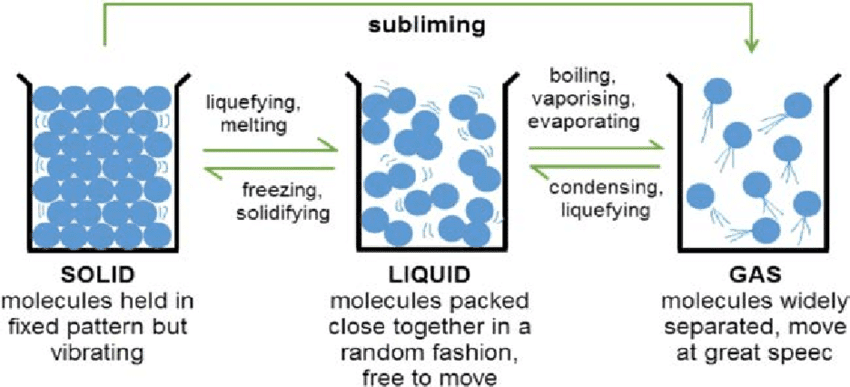
- Particles are tightly packed and vibrate in fixed positions
- Maintains a definite shape and volume
- Examples: Ice, wood, metals, crystals
Liquid State:
- Picture water flowing from a tap or juice in a glass
- Particles are close together but can move around each other
- Takes the shape of its container but maintains definite volume
- Examples: Water, oil, mercury, liquid nitrogen
Gas State:
- Imagine the air you’re breathing or steam from boiling water
- Particles are far apart and move freely in all directions
- No definite shape or volume – expands to fill any container
- Examples: Oxygen, carbon dioxide, water vapor, helium
The Kinetic Particle Theory – Your Key to Understanding Everything
Here’s where things get really interesting! The kinetic particle theory is like having X-ray vision into the invisible world of atoms and molecules. This theory explains why matter behaves the way it does, and once you understand it, chemistry becomes so much clearer.
The Five Key Principles:
- All matter is made up of tiny particles (atoms, molecules, or ions)
- These particles are in constant motion (even in solids!)
- The higher the temperature, the faster the particles move
- Particles attract each other (some more strongly than others)
- The kinetic energy of particles increases with temperature
Memory Tip: Remember “PACE” – Particles Always Constantly Energetic!
H2: Diving Deeper – Properties of Each State
Solids – The Organized State
Imagine a perfectly organized classroom where every student sits in their assigned seat – that’s essentially what a solid is like at the particle level!
Key Characteristics:
- Definite shape and volume – Won’t change unless you apply force
- High density – particles are packed closely together
- Incompressible – you can’t squeeze them into smaller spaces
- Particles vibrate about fixed positions – like students wiggling in their seats
Real-Life Applications:
- Building materials (concrete, steel, wood)
- Electronic components in your devices
- The structure of diamonds and graphite (we’ll explore this more in later topics!)
Liquids – The Flexible Middle Ground
If solids are like organized classrooms, liquids are like a busy cafeteria where people can move around but still stay relatively close together.
Key Characteristics:
- Definite volume but no definite shape – takes the shape of its container
- Medium density – less dense than solids, more dense than gases
- Slightly compressible – but not by much
- Particles can move past each other – allowing flow
Why Liquids Flow:
The secret is in the particle movement! While particles in liquids are still attracted to each other, they have enough energy to overcome some of these attractions and slide past one another. It’s like a gentle dance where partners can switch but never stray too far apart.
Everyday Examples:
- Blood flowing through your veins
- Rivers and ocean currents
- The ink in your pen
- Liquid cooling systems in computers
Gases – The Free Spirits
Gases are like particles at a music festival – spread out, moving freely, and filling up all available space!
Key Characteristics:
- No definite shape or volume – expands to fill any container completely
- Low density – particles are far apart
- Highly compressible – lots of empty space between particles
- Particles move rapidly in random directions
Why Gases Behave This Way:
In gases, particles have so much kinetic energy that they’ve overcome most of the attractive forces between them. They zoom around like tiny racecars, only occasionally bumping into each other or the container walls.
Fascinating Gas Facts:
- The air in your classroom contains about 25 quintillion molecules per cubic centimeter!
- Gas particles move at hundreds of meters per second
- Despite all this movement, gases mix evenly due to diffusion
State Changes – The Magic of Phase Transitions
Understanding State Changes Through Energy
Here’s where chemistry gets really exciting! State changes aren’t just about heating or cooling – they’re about energy battles between particle movement and particle attractions.
The Energy Perspective:
- Add energy (heat): Particles move faster, overcome attractions, change to less ordered state
- Remove energy (cool): Particles slow down, attractions win, change to more ordered state
The Six State Changes You Must Know
1. Melting (Solid → Liquid)
- Process: Adding heat energy to overcome rigid structure
- Example: Ice cube melting in your drink
- Key Point: Temperature remains constant during melting (melting point)
2. Freezing (Liquid → Solid)
- Process: Removing heat energy, particles lock into rigid positions
- Example: Water forming ice in your freezer
- Key Point: Occurs at the same temperature as melting (freezing point)
3. Boiling (Liquid → Gas)
- Process: Adding enough energy for particles to completely escape liquid
- Example: Water boiling in a kettle
- Key Point: Occurs at a specific temperature (boiling point)
4. Condensation (Gas → Liquid)
- Process: Removing energy, gas particles slow down and come together
- Example: Steam condensing on a cold window
- Key Point: Opposite of boiling
5. Sublimation (Solid → Gas)
- Process: Direct change from solid to gas without melting
- Example: Dry ice (solid CO₂) disappearing
- Key Point: Skips the liquid phase entirely!
6. Deposition (Gas → Solid)
- Process: Direct change from gas to solid
- Example: Frost forming on grass on cold mornings
- Key Point: Opposite of sublimation
Heating and Cooling Curves – Reading the Energy Story
Heating curves are like energy fingerprints that tell the complete story of what happens when you heat a substance from solid to gas.
Key Features of Heating Curves:
- Sloped sections: Temperature increases as you add energy
- Flat sections: Temperature stays constant during state changes
- The flat sections show: All energy goes into breaking intermolecular forces, not increasing temperature
Why This Matters for Exams:
Understanding heating curves helps you explain why ice-cold drinks stay cold longer and why cooking times vary at different altitudes!
Factors Affecting State Changes
Temperature – The Primary Driver
Temperature is essentially a measure of average particle kinetic energy. Higher temperature = faster particles = more likely to overcome attractions and change state.
Key Temperature Points:
- Melting Point: Temperature where solid becomes liquid
- Boiling Point: Temperature where liquid becomes gas
- These are specific to each substance and can be used for identification
Pressure – The Often Forgotten Factor
Pressure significantly affects boiling points but has minimal effect on melting points.
Pressure Effects:
- Higher pressure: Increases boiling point (harder for particles to escape)
- Lower pressure: Decreases boiling point (easier for particles to escape)
- Real Example: Water boils at lower temperatures on mountains due to lower atmospheric pressure
Nature of the Substance – Why Different Materials Behave Differently
The strength of forces between particles determines the temperatures at which state changes occur.
Types of Intermolecular Forces (from weakest to strongest):
- Van der Waals forces (very weak)
- Dipole-dipole attractions (moderate)
- Hydrogen bonding (strong)
Practical Impact:
- Substances with weaker forces have lower melting and boiling points
- Substances with stronger forces require more energy to change state
Real-World Applications and Examples
The Water Cycle – States of Matter in Action
The water cycle is probably the best real-world example of states of matter working together:
- Evaporation: Liquid water becomes water vapor (gas)
- Condensation: Water vapor becomes liquid droplets in clouds
- Precipitation: Liquid droplets fall as rain
- Freezing: Water becomes ice in cold conditions
- Melting: Ice returns to liquid water
Industrial Applications
Distillation:
- Uses different boiling points to separate mixtures
- Essential in oil refining and alcohol production
Freeze-Drying:
- Uses sublimation to preserve food and medicines
- Removes water while maintaining structure
Refrigeration:
- Uses state changes of refrigerant to transfer heat
- The coolant evaporates (absorbing heat) and condenses (releasing heat)
Common Exam Questions and How to Tackle Them
Typical Question Types
1. Describe and Explain Questions:
- “Describe what happens to particles when a solid melts”
- Approach: Always mention both particle arrangement AND movement
2. Graph Interpretation:
- “Explain the shape of this heating curve”
- Key: Identify flat sections as state changes, sloped sections as temperature increases
3. Comparison Questions:
- “Compare the properties of solids and gases”
- Strategy: Use a systematic approach covering shape, volume, density, compressibility
4. Application Questions:
- “Explain why cooking takes longer at high altitudes”
- Method: Link to pressure effects on boiling point
Model Answers and Approach
Sample Question: “Explain, in terms of particles, what happens when ice melts.”
Model Answer Structure:
- State change identified: Ice (solid) changes to water (liquid)
- Energy input: Heat energy is added
- Particle behavior: Particles gain kinetic energy and vibrate more vigorously
- Force changes: Overcome some attractive forces between particles
- Result: Particles can move past each other while remaining close together
Key Formulas and Equations Box
Essential Relationships:
- Kinetic Energy ∝ Temperature (Higher temperature = more kinetic energy)
- Pressure × Volume = Constant (for gases at constant temperature)
- Density = Mass ÷ Volume
State Change Equations:
- Heat energy required = mass × specific heat capacity × temperature change
- For state changes: Heat energy = mass × latent heat
Gas Laws (Introduction):
- Charles’s Law: Volume ∝ Temperature (at constant pressure)
- Boyle’s Law: Pressure ∝ 1/Volume (at constant temperature)
Quick Revision Notes
Essential Facts for Quick Review
States of Matter Summary:
- Solids: Fixed shape and volume, particles vibrate in fixed positions
- Liquids: Fixed volume, variable shape, particles can move past each other
- Gases: Variable shape and volume, particles move freely
State Changes:
- Melting/Freezing: Solid ↔ Liquid
- Boiling/Condensation: Liquid ↔ Gas
- Sublimation/Deposition: Solid ↔ Gas
Key Factors:
- Temperature: Primary driver of state changes
- Pressure: Affects boiling point significantly
- Intermolecular forces: Determine ease of state changes
Memory Techniques
SOLID Memory Aid: Structured Organized Locked In Definite positions
LIQUID Memory Aid: Loose Interactions Quickly Undergoing Internal Dancing
GAS Memory Aid: Greatly Active Spaced-out particles
Common Mistakes to Avoid
- Don’t confuse heat with temperature – Heat is energy transfer, temperature is a measure of particle kinetic energy
- Remember that particles never stop moving – Even in solids, they vibrate
- Don’t forget that boiling point changes with pressure – It’s not always 100°C for water
- Sublimation isn’t just for dry ice – Many substances can sublime under right conditions
Test Yourself – Practice Questions
Quick Check Questions:
- What happens to particle movement when you heat a solid?
- Why do gases fill their containers completely?
- Explain why water boils at a lower temperature on Mount Everest.
- What type of state change occurs when frost forms on grass?
Challenge Questions:
- A student observes that perfume spreads across a room. Explain this in terms of particle theory.
- Compare the density of the same substance in solid, liquid, and gas states.
- Explain why the temperature remains constant during melting, even though heat is being added.
Your Next Steps to Chemistry Success
Building on This Foundation
Congratulations! You’ve just mastered one of the most fundamental topics in IGCSE Chemistry. This knowledge will serve as the foundation for understanding:
- Topic 2: Atomic Structure – How particle behavior relates to atomic structure
- Topic 8: Acids, Bases and Salts – How ionic compounds behave in different states
- Topic 10: Metals – Understanding metallic bonding and properties
- Topic 11: Air and Water – Real-world applications of state changes
Stay Motivated and Keep Learning
Remember, every chemistry expert started exactly where you are now. The concepts that seem challenging today will become second nature with practice and application. States of matter is everywhere around you – use this to your advantage by constantly observing and explaining the world through your new particle theory lens.
You’ve got this! Chemistry is not about memorizing facts; it’s about understanding the beautiful, logical patterns that govern our universe. Keep curious, keep questioning, and most importantly, keep connecting what you learn to the amazing world around you.
Your chemistry journey has just begun, and Topic 1 is your solid foundation for everything amazing that’s coming next!
Ready to dive deeper? Explore our complete IGCSE Chemistry series covering all topics with the same engaging, student-friendly approach. Next up: Atomic Structure – where we’ll discover what those particles are actually made of!
Recommended –


2 thoughts on “IGCSE (Cambridge) Chemistry Topic 1: States of Matter | Your Complete Study Guide”