The Building Blocks of Everything Around You
Have you ever wondered what makes up the water you drink, the air you breathe, or even your own body? The answer lies in understanding atoms, elements, and compounds – the fundamental building blocks of matter. Think of atoms as LEGO blocks: just as you can build countless structures with different combinations of LEGO pieces, nature creates everything around us by combining atoms in different ways.
This topic forms the foundation of all chemistry, and mastering it will make every other chemistry concept much easier to understand. Whether you’re aiming for that A* grade or just trying to make sense of chemical reactions, this guide will take you from confusion to confidence.
What Are Atoms? The Invisible Building Blocks
Understanding Atomic Structure
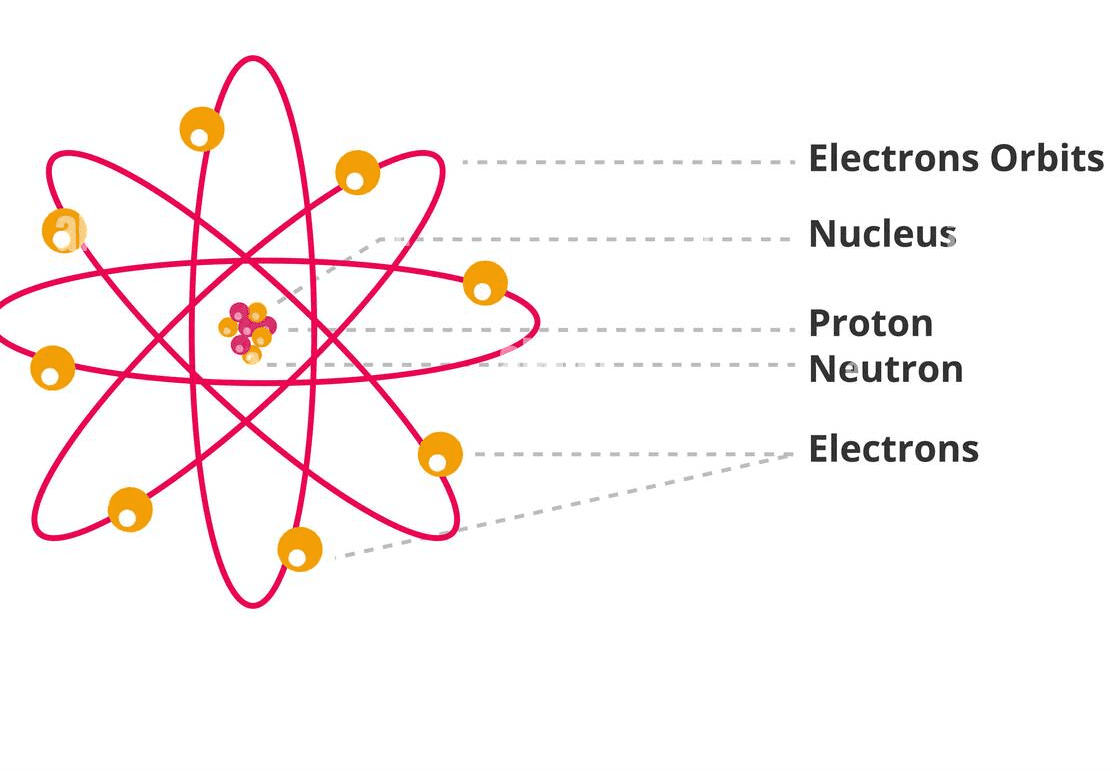
An atom is the smallest unit of matter that still retains the properties of an element. Imagine trying to cut a piece of gold into smaller and smaller pieces – eventually, you’d reach a point where you have the tiniest possible piece that’s still gold. That’s an atom!
Key Components of an Atom:
Nucleus (The Core):
- Contains protons (positive charge)
- Contains neutrons (no charge)
- Extremely small but contains almost all the atom’s mass
- Think of it as the sun in our solar system
Electrons (The Dancers):
- Negatively charged particles
- Orbit around the nucleus in shells/energy levels
- Determine how atoms bond with each other
- Like planets orbiting the sun, but in 3D shells
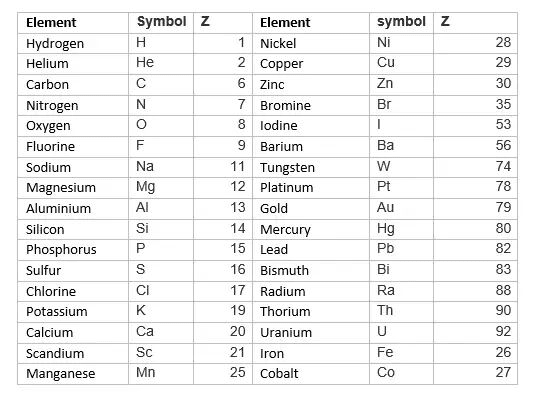
Atomic Number and Mass Number
Atomic Number (Z):
- Number of protons in the nucleus
- Defines what element it is
- Never changes for a given element
- Also equals the number of electrons in a neutral atom
Mass Number (A):
- Total number of protons + neutrons
- Can vary for the same element (isotopes)
- Always a whole number
Memory Tip: Remember “PEN” – Protons determine the Element Name!
Isotopes: Same Element, Different Mass
Isotopes are atoms of the same element with different numbers of neutrons. Think of them as identical twins with different weights – they’re still the same person (element) but have slight differences.
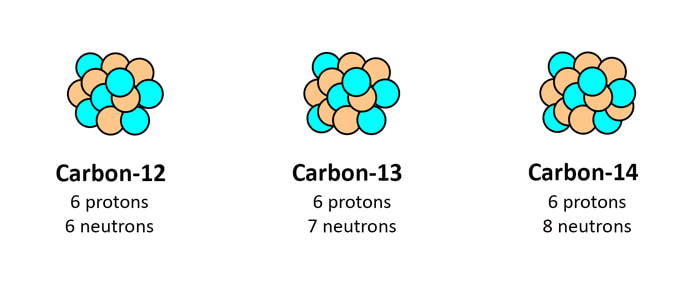
Example: Carbon-12 and Carbon-14
- Both have 6 protons (that’s what makes them carbon)
- Carbon-12 has 6 neutrons
- Carbon-14 has 8 neutrons
Real-life Application: Carbon-14 is used in radiocarbon dating to determine the age of ancient objects!
Elements: The Pure Substances
What Makes an Element Special?
An element is a pure substance that cannot be broken down into simpler substances by chemical means. It contains only one type of atom. Think of elements as the “alphabet” of chemistry – just as we combine letters to make words, we combine elements to make compounds.
The Periodic Table: Your Chemical Map
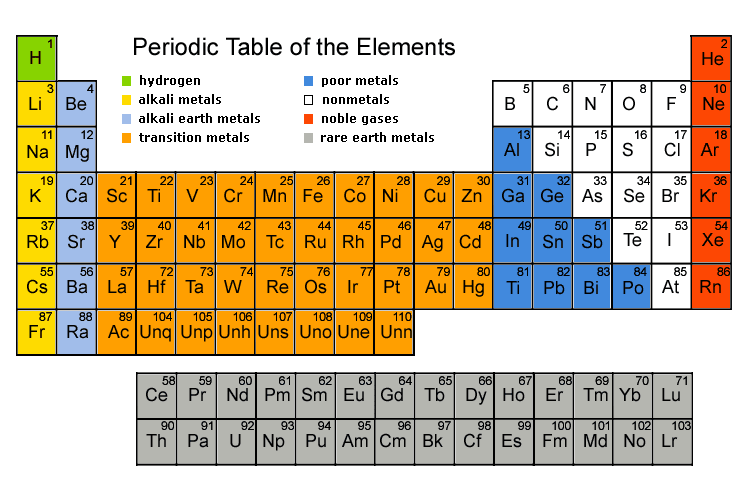
The periodic table isn’t just a random arrangement – it’s organized by increasing atomic number and shows patterns in properties.
Key Features:
- Periods (horizontal rows): Elements have the same number of electron shells
- Groups (vertical columns): Elements have similar chemical properties
- Metals: Left side and center (good conductors, shiny, malleable)
- Non-metals: Right side (poor conductors, brittle when solid)
- Metalloids: Along the “staircase” line (properties between metals and non-metals)
Group Properties to Remember:
- Group 1 (Alkali metals): Very reactive, soft metals
- Group 7 (Halogens): Very reactive non-metals
- Group 0/8 (Noble gases): Unreactive gases
Electron Configuration: The Address System
Electrons live in shells around the nucleus, like floors in a building. Each shell can hold a maximum number of electrons:
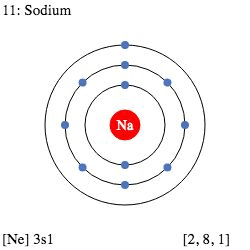
- 1st shell: maximum 2 electrons
- 2nd shell: maximum 8 electrons
- 3rd shell: maximum 8 electrons (for first 20 elements)
Example: Sodium (Na) has 11 electrons
Configuration: 2, 8, 1 (2 in first shell, 8 in second, 1 in third)
Why This Matters: The number of electrons in the outer shell determines how elements react!
Compounds: When Elements Team Up
Formation of Compounds
A compound is a substance made from two or more different elements chemically combined in fixed proportions. Think of it like a recipe – water is always H₂O, never H₃O or HO₂.
Key Points:
- Properties are completely different from the original elements
- Can only be separated by chemical methods
- Always have the same formula and composition
Amazing Example: Sodium (explosive metal) + Chlorine (poisonous gas) = Sodium chloride (table salt we eat!)
Types of Chemical Bonding
Ionic Bonding: The Give and Take
Occurs between metals and non-metals through electron transfer.
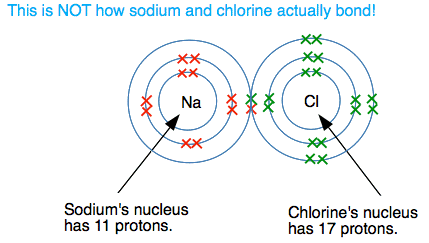
How it works:
- Metal atoms lose electrons to become positive ions (cations)
- Non-metal atoms gain electrons to become negative ions (anions)
- Opposite charges attract, forming ionic bonds
Example: Sodium chloride (NaCl)
- Na loses 1 electron → Na⁺
- Cl gains 1 electron → Cl⁻
- Na⁺ and Cl⁻ attract each other
Properties of Ionic Compounds:
- High melting and boiling points
- Conduct electricity when dissolved or molten
- Often soluble in water
- Form crystal structures
Covalent Bonding: The Sharing Economy
Occurs between non-metals through electron sharing.
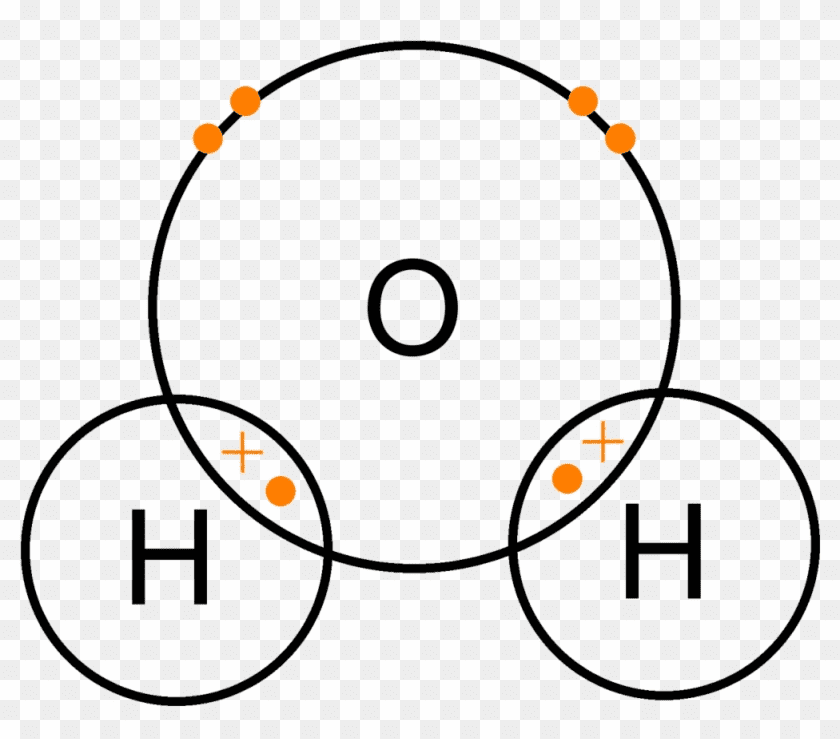
How it works:
- Non-metal atoms share pairs of electrons
- Shared electrons are attracted to both nuclei
- Forms molecules
Example: Water (H₂O)
- Each hydrogen shares 1 electron with oxygen
- Oxygen shares 1 electron with each hydrogen
- Everyone gets a full outer shell!
Properties of Covalent Compounds:
- Lower melting and boiling points (usually)
- Don’t conduct electricity
- May be soluble in non-polar solvents
Chemical Formulas: The Language of Chemistry
Chemical formulas tell us exactly what’s in a compound and how much of each element.
Reading Formulas:
- H₂O: 2 hydrogen atoms, 1 oxygen atom
- CaCl₂: 1 calcium atom, 2 chlorine atoms
- Mg(OH)₂: 1 magnesium, 2 oxygen, 2 hydrogen atoms
Brackets in Formulas:
When you see brackets like Ca(OH)₂, multiply everything inside the brackets by the number outside.
Writing Chemical Formulas
For Ionic Compounds:
- Write the metal first, then the non-metal
- Use the charges to determine the ratio
- Swap and drop the charges (ignore signs)
Example: Aluminum oxide
- Al³⁺ and O²⁻
- Al₂O₃ (swap and drop: Al gets 2, O gets 3)
Common Ion Charges to Remember:
- Group 1: +1 (Li⁺, Na⁺, K⁺)
- Group 2: +2 (Mg²⁺, Ca²⁺)
- Group 7: -1 (F⁻, Cl⁻, Br⁻, I⁻)
- Group 6: -2 (O²⁻, S²⁻)
Mixtures vs Compounds: Spot the Difference
Mixtures:
- Components keep their own properties
- Can be separated by physical methods
- No fixed composition
- No chemical bonds
Examples: Air, seawater, oil and water
Compounds:
- New properties, different from elements
- Can only be separated by chemical methods
- Fixed composition
- Chemical bonds present
Examples: Water, salt, carbon dioxide
Separation Methods for Mixtures:
- Filtration: Separating insoluble solids from liquids
- Distillation: Separating liquids with different boiling points
- Chromatography: Separating colored substances
- Crystallization: Obtaining pure solid from solution
Key Formulas and Equations Box
Atomic Structure:
- Mass Number (A) = Protons + Neutrons
- Atomic Number (Z) = Number of Protons = Number of Electrons (in neutral atom)
Electron Configuration:
- Maximum electrons in shells: 2, 8, 8 (for first 20 elements)
Relative Atomic Mass:
- Weighted average of isotopes
Formula Writing Rules:
- Metal + Non-metal = Ionic compound
- Non-metal + Non-metal = Covalent compound
- Charges must balance in ionic compounds
Common Mistakes to Avoid
- Confusing atoms and molecules: An atom is a single particle; a molecule is two or more atoms bonded together
- Mixing up mass number and atomic number: Remember – atomic number is smaller and defines the element
- Forgetting brackets in formulas: Ca(OH)₂ has 2 OH groups, not CaO₂H₂
- Not balancing charges in ionic compounds: Always check that positive and negative charges cancel out
- Assuming all compounds are ionic: Remember, non-metal + non-metal = covalent!
Memory Tips and Mnemonics
For Group Names:
- “All Good Boys Do Fine” (Groups 1,2,7,0: Alkali, alkaline earth, halogens, noble Gases)
For Electron Shells:
- “2-8-8 rule” – like a bus route number!
For Ion Charges:
- “1-2-3-1-2” across periods 2&3 (how many electrons to lose/gain for full outer shell)
For Metals vs Non-metals:
- Metals are “generous” (give electrons away)
- Non-metals are “greedy” (take electrons)
Test Yourself: Quick Check Questions
- What is the atomic number of an element with 15 protons and 16 neutrons?
- How many electrons does Mg²⁺ have if magnesium has 12 protons?
- What type of bonding occurs in CO₂?
- Write the formula for calcium chloride.
Answers:
- 15 (atomic number = number of protons)
- 10 electrons (12 – 2 = 10, lost 2 electrons to form 2+ ion)
- Covalent (both carbon and oxygen are non-metals)
- CaCl₂ (Ca²⁺ and Cl⁻, need 2 chlorines to balance)
Quick Revision Notes
Essential Facts to Remember:
- Atom: Smallest unit of an element
- Element: Pure substance, one type of atom only
- Compound: Two or more elements chemically combined
- Mixture: Two or more substances physically combined
- Ionic bonding: Metal + Non-metal, electron transfer, high melting points
- Covalent bonding: Non-metal + Non-metal, electron sharing, lower melting points
- Atomic number = Protons = Electrons (neutral atom)
- Mass number = Protons + Neutrons
- Electron shells: Maximum 2, 8, 8 for first 20 elements
- Isotopes: Same protons, different neutrons
- Formula writing: Balance charges for ionic compounds
- Group properties: Vertical columns have similar properties
Important Exam Questions to Practice
Foundation Level:
- Define the terms: atom, element, compound, mixture
- State the particles found in the nucleus of an atom
- An atom has 19 protons and 20 neutrons. What is its mass number?
- Give the electron configuration of chlorine (atomic number 17)
Higher Level:
- Explain why isotopes of the same element have identical chemical properties
- Compare the properties of ionic and covalent compounds
- Explain how ionic bonding occurs between sodium and chlorine
- A compound has the formula Ca₃(PO₄)₂. How many atoms of each element are present?
Extended Response:
- Describe the differences between mixtures and compounds, giving examples
- Explain why noble gases are unreactive in terms of their electron configuration
Your Next Steps to Chemistry Success
Congratulations! You’ve just covered one of the most important topics in IGCSE Chemistry. Understanding atoms, elements, and compounds is like learning the alphabet before reading – it makes everything else possible.
What to Study Next:
- Topic 3: Stoichiometry – How to calculate quantities in chemical reactions
- Topic 4: Electrochemistry – How electrons create electric currents
- Topic 5: Chemical Energetics – Energy changes in reactions
Study Tips for Continued Success:
- Practice drawing: Atomic diagrams, electron configurations, bonding diagrams
- Make flashcards: Element symbols, ion charges, key definitions
- Connect concepts: Link this topic to chemical reactions and the periodic table
- Use past papers: Practice exam-style questions regularly
- Form study groups: Explain concepts to classmates – teaching helps learning!
Remember: Every chemistry expert started exactly where you are now. The key is consistent practice and not being afraid to ask questions. You’ve got this!
Final Motivation: Chemistry isn’t just about memorizing facts – you’re learning to understand the very fabric of reality. Every breath you take, every bite of food you eat, every medicine that heals you – it’s all chemistry. You’re not just studying for an exam; you’re unlocking the secrets of the universe!
Keep practicing, stay curious, and remember that every mistake is just a step closer to mastery. Your future self will thank you for the effort you put in today. Good luck with your IGCSE Chemistry journey!
Ready to dive deeper? Check out our other IGCSE Chemistry guides and practice tests to boost your confidence even further!
Recommended –

1 thought on “IGCSE (Cambridge) Chemistry Topic 2: Atoms, Elements and Compounds | Your Complete Study Guide”