Why Electrochemistry Powers Our World
Picture this: you wake up, check your smartphone (powered by a lithium-ion battery), brush your teeth with an electric toothbrush, and maybe your parents start their car with an electric starter. All of these everyday miracles are possible because of electrochemistry!
As an IGCSE Chemistry student, you’re about to discover one of the most practical and fascinating topics in your syllabus. Electrochemistry isn’t just about passing exams – it’s the science behind batteries, fuel cells, metal plating, and even the process that prevents ships from rusting. By the end of this guide, you’ll not only ace your Topic 4 questions but also see the world around you with new scientific eyes.
Don’t worry if the topic seems daunting at first. We’ll break down every concept into bite-sized, understandable chunks. Remember, every chemistry expert started exactly where you are now!
What is Electrochemistry? The Foundation You Need
Electrochemistry is simply the study of chemical reactions that involve the transfer of electrons and the relationship between chemical energy and electrical energy. Think of it as the bridge between chemistry and electricity.
Key Concepts to Master
Oxidation and Reduction (Redox Reactions)
- Oxidation: Loss of electrons (think “OIL” – Oxidation Is Loss)
- Reduction: Gain of electrons (think “RIG” – Reduction Is Gain)
- Memory Tip: “OILRIG” – a simple way to remember both processes!
Oxidation States (Oxidation Numbers)
These numbers help us track electron transfer in reactions. Here are the essential rules:
- Elements in their pure state have oxidation state 0
- Group 1 metals always have +1
- Group 2 metals always have +2
- Oxygen usually has -2 (except in peroxides)
- Hydrogen usually has +1
Real-Life Connection
When you bite into an apple and it turns brown, that’s oxidation in action! The iron in the apple reacts with oxygen in the air. Similarly, when you see rust on a bicycle, iron is being oxidized. Understanding these processes helps us prevent unwanted reactions and harness useful ones.
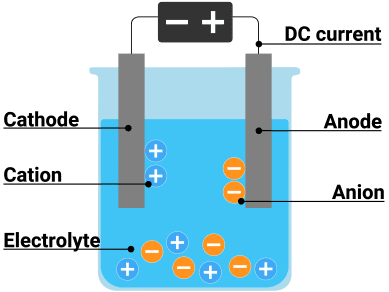
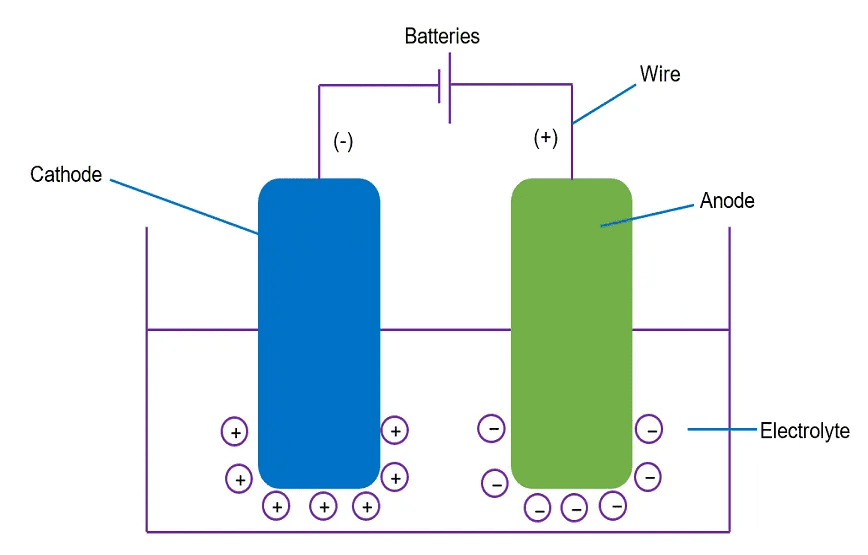
Electrolytic Cells – Making Chemistry Happen with Electricity
Electrolytic cells use electrical energy to drive chemical reactions that wouldn’t happen spontaneously. It’s like forcing a ball to roll uphill!
Components of an Electrolytic Cell
- Electrolyte: A substance that conducts electricity when molten or dissolved
- Electrodes: Conductors where reactions occur
- Anode: Positive electrode where oxidation occurs
- Cathode: Negative electrode where reduction occurs
- Power Source: Provides the electrical energy
Memory Trick: “AN OX and RED CAT”
- ANode = OXidation
- REDuction = CAThode
Electrolysis of Water – A Classic Example
When we electrolyze water (H₂O), here’s what happens:
At the Cathode (-): 2H⁺ + 2e⁻ → H₂ (hydrogen gas produced)
At the Anode (+): 4OH⁻ → O₂ + 2H₂O + 4e⁻ (oxygen gas produced)
Overall Reaction: 2H₂O → 2H₂ + O₂
Industrial Applications You Should Know
- Electroplating: Coating objects with thin layers of metals (like chrome on car bumpers)
- Metal Extraction: Obtaining pure metals from their ores (aluminum from bauxite)
- Purification: Refining copper to 99.9% purity
Galvanic (Voltaic) Cells – Chemistry Creating Electricity
Unlike electrolytic cells, galvanic cells generate electrical energy from spontaneous chemical reactions. Your phone battery is a sophisticated galvanic cell!
How Galvanic Cells Work
In a galvanic cell:
- Chemical energy converts to electrical energy
- Reactions occur spontaneously
- Electrons flow from anode to cathode through an external circuit
Classic Example: Zinc-Copper Cell
At the Zinc Anode: Zn → Zn²⁺ + 2e⁻ (oxidation)
At the Copper Cathode: Cu²⁺ + 2e⁻ → Cu (reduction)
Overall: Zn + Cu²⁺ → Zn²⁺ + Cu
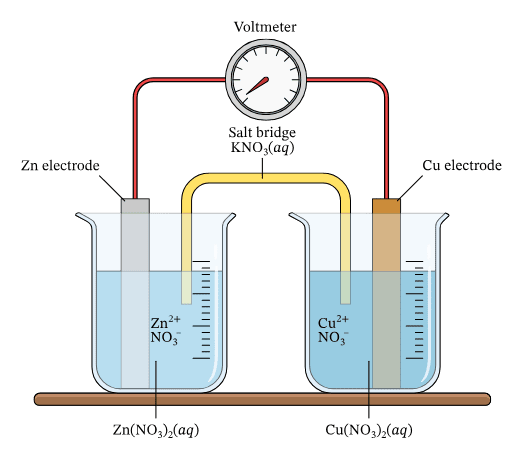
The Salt Bridge – The Unsung Hero
The salt bridge maintains electrical neutrality by allowing ions to flow between the two half-cells. Without it, the reaction would quickly stop due to charge buildup.
Common Salt Bridge Solutions:
- Potassium nitrate (KNO₃)
- Sodium chloride (NaCl)
- Potassium chloride (KCl)
Electrode Potentials – Predicting What Will Happen
Standard electrode potentials help us predict which reactions will occur spontaneously and calculate cell voltages.
Understanding the Electrochemical Series
The electrochemical series ranks elements by their tendency to lose electrons:
Most Reactive (Most Likely to Lose Electrons)
- Potassium (K)
- Sodium (Na)
- Magnesium (Mg)
- Aluminum (Al)
- Zinc (Zn)
- Iron (Fe)
- Hydrogen (H)
- Copper (Cu)
- Silver (Ag)
- Gold (Au)
Least Reactive (Least Likely to Lose Electrons)
Calculating Cell Potentials
Formula: E°(cell) = E°(cathode) – E°(anode)
For a spontaneous reaction, E°(cell) must be positive.
Example Calculation:
Zn²⁺/Zn = -0.76V
Cu²⁺/Cu = +0.34V
E°(cell) = (+0.34) – (-0.76) = +1.10V
Since this is positive, the reaction is spontaneous!
Factors Affecting Electrolysis
Several factors influence what happens during electrolysis:
Nature of the Electrolyte
Aqueous Solutions: Water competes with other substances
- At the cathode: Less reactive metals are deposited; more reactive metals stay in solution
- At the anode: Non-metals are often discharged; metals remain as ions
Molten Compounds: Only the compound itself is electrolyzed
- Simpler to predict products
- Higher temperatures required
Concentration Effects
Higher concentrations favor the discharge of that particular ion. This is why concentrated sodium chloride gives chlorine gas, while dilute solutions give oxygen.
Electrode Material
Inert Electrodes (platinum, carbon): Don’t participate in reactions
Reactive Electrodes (copper, silver): Can participate in reactions
Practical Applications in Everyday Life
Batteries – Portable Power
Primary Cells (non-rechargeable):
- Dry cells (zinc-carbon)
- Alkaline batteries
- Lithium batteries
Secondary Cells (rechargeable):
- Lead-acid car batteries
- Lithium-ion phone batteries
- Nickel-metal hydride batteries
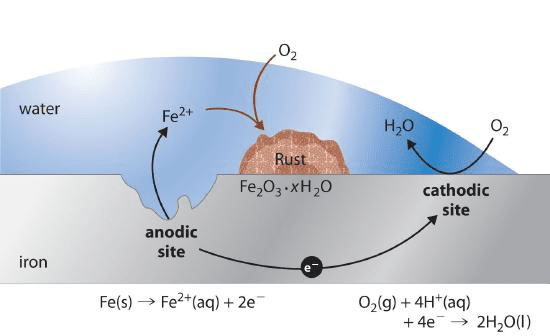
Corrosion and Prevention
Rusting of Iron:
Fe → Fe²⁺ + 2e⁻ (oxidation at anodic areas)
O₂ + 4H⁺ + 4e⁻ → 2H₂O (reduction at cathodic areas)
Prevention Methods:
- Galvanizing: Coating with zinc
- Sacrificial Protection: Attaching more reactive metals
- Cathodic Protection: Making iron the cathode
Common Exam Questions and How to Tackle Them
Electrolysis Prediction Questions
Question Type: “Predict the products of electrolyzing concentrated sodium chloride solution using inert electrodes.”
Strategy:
- Identify possible ions present
- Consider electrode reactions
- Apply rules for aqueous solutions
- Write balanced equations
Answer:
- Cathode: 2H⁺ + 2e⁻ → H₂
- Anode: 2Cl⁻ → Cl₂ + 2e⁻
Cell Potential Calculations
Question Type: “Calculate the EMF of a cell made from silver and zinc electrodes.”
Strategy:
- Identify which is anode and cathode
- Look up standard potentials
- Apply formula: E° = E°(cathode) – E°(anode)
- Check if reaction is spontaneous
Redox Equation Balancing
Steps to Balance:
- Write separate half-equations
- Balance atoms other than H and O
- Balance oxygen using H₂O
- Balance hydrogen using H⁺
- Balance charge using electrons
- Multiply to equalize electrons
- Add equations and simplify
Quick Revision Box
Key Formulas and Equations
Cell EMF: E°(cell) = E°(cathode) – E°(anode)
Faraday’s Laws:
- Amount of substance produced ∝ charge passed
- Q = I × t (charge = current × time)
Key Reactions to Remember:
- Electrolysis of water: 2H₂O → 2H₂ + O₂
- Electrolysis of brine: 2NaCl + 2H₂O → H₂ + Cl₂ + 2NaOH
Memory Aids
- OILRIG: Oxidation Is Loss, Reduction Is Gain
- AN OX, RED CAT: Anode Oxidation, Reduction Cathode
- LEO says GER: Lose Electrons Oxidation, Gain Electrons Reduction
Common Mistakes to Avoid
- Confusing electrode names: Remember anode is always where oxidation occurs
- Forgetting competition in aqueous solutions: Water can compete with other substances
- Wrong electron flow direction: Electrons flow from anode to cathode in external circuit
- Ignoring spectator ions: Not all ions participate in electrode reactions
Test Yourself – Practice Questions
- Basic Level: What are the products of electrolyzing molten sodium chloride?
- Intermediate Level: Calculate the EMF of a galvanic cell with copper and aluminum electrodes. (Cu²⁺/Cu = +0.34V, Al³⁺/Al = -1.66V)
- Advanced Level: Explain why silver objects are often electroplated with gold, including the electrode reactions involved.
- Application Level: A student wants to electroplate a steel spoon with copper. Draw and label the setup, identifying which electrode the spoon should be connected to and why.
Study Tips for Exam Success
Understanding vs. Memorizing
Focus on understanding the underlying principles rather than memorizing equations. When you understand why electrons flow from zinc to copper, you’ll never forget which is the anode!
Practice with Past Papers
Electrochemistry questions often follow similar patterns. Practice identifying:
- Products of electrolysis
- Direction of electron flow
- Calculating cell potentials
- Predicting spontaneous reactions
Connect to Real Life
Make connections between theory and applications. When you see a car battery, think about the lead-acid reactions happening inside. This makes the subject more memorable and interesting!
Your Next Steps to Mastery
Congratulations! You’ve just completed a comprehensive journey through IGCSE Chemistry Topic 4. You now understand how chemical reactions can generate electricity and how electricity can drive chemical changes.
Immediate Action Plan:
- Review the key formulas in the revision box above
- Practice the test questions until you can answer them confidently
- Make flashcards for the electrochemical series
- Draw diagrams from memory to reinforce visual learning
Related Topics to Explore:
- Topic 3: Chemical Bonding – Understanding how electrons behave in compounds
- Topic 5: Chemical Energetics – How energy changes relate to electrochemical processes
- Topic 8: Acids and Bases – pH changes in electrolytic solutions
Exam Preparation Strategy:
Start practicing past paper questions now, even if you haven’t finished the entire syllabus. Electrochemistry questions often combine with other topics, so early practice helps you see these connections.
Remember, every expert was once a beginner. The scientists who developed our understanding of electrochemistry faced the same challenges you’re facing now. With consistent practice and the right approach, you’ll not only master this topic but also develop a deeper appreciation for the elegant way chemistry and physics work together.
You’ve got this! Your future self will thank you for the effort you put in today. Keep asking questions, keep practicing, and most importantly, keep that curiosity alive. Science belongs to those who never stop wondering “how” and “why.”
Good luck with your studies, and remember – you’re not just learning chemistry, you’re discovering the fundamental principles that power our modern world!
Recommended –


2 thoughts on “IGCSE (Cambridge) Chemistry Topic 4: Electrochemistry | Your Complete Study Guide”