Why Energy Matters in Chemistry
Imagine striking a match – within seconds, a tiny wooden stick transforms into light, heat, and ash. Or think about the ice pack you use for injuries that gets cold when you squeeze it. These everyday phenomena are perfect examples of chemical energetics in action!
Chemical energetics is essentially the study of energy changes that occur during chemical reactions. It’s like being an energy detective, investigating how and why reactions either release energy (making things hot) or absorb energy (making things cold). This topic forms the backbone of understanding how chemical processes work in everything from our bodies to industrial manufacturing.
For IGCSE Chemistry students, Topic 5 is often considered challenging, but don’t worry – we’re going to break it down into digestible pieces that will have you solving energy problems with confidence!
What Exactly Is Chemical Energetics?
Chemical energetics deals with the energy changes that accompany chemical reactions. Every chemical reaction involves either the release or absorption of energy, usually in the form of heat. Think of it as the “energy currency” of chemical reactions.
When atoms bond together or break apart, energy is either released to the surroundings or absorbed from them. This is because bonds have different strengths – some require more energy to break than others, and some release more energy when formed.
Key Point: Energy cannot be created or destroyed (Law of Conservation of Energy), but it can be transferred from one form to another during chemical reactions.
Understanding Enthalpy Changes (ΔH)
What Is Enthalpy?
Enthalpy (H) is the total heat content of a system. Since we can’t measure absolute enthalpy, we focus on enthalpy changes (ΔH) – the difference in heat content between products and reactants.
Formula: ΔH = H(products) – H(reactants)
Types of Enthalpy Changes
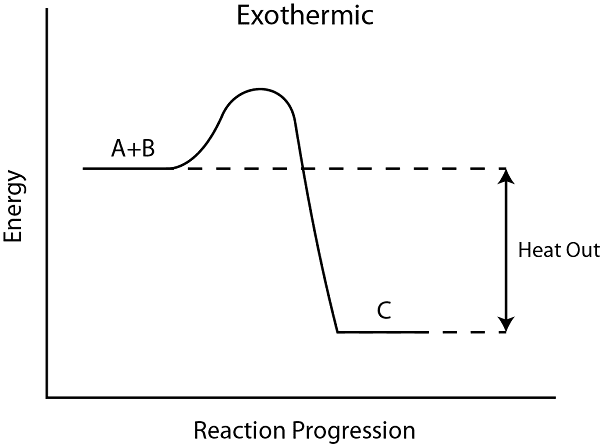
1. Exothermic Reactions (ΔH is negative)
- Energy is released to the surroundings
- Products have less energy than reactants
- Temperature of surroundings increases
- Examples: Combustion, neutralization, respiration
2. Endothermic Reactions (ΔH is positive)
- Energy is absorbed from the surroundings
- Products have more energy than reactants
- Temperature of surroundings decreases
- Examples: Photosynthesis, thermal decomposition of carbonates
Memory Tip: “EXO = EXIT” (energy exits the reaction), “ENDO = ENTER” (energy enters the reaction)
Standard Enthalpy Changes
The IGCSE syllabus focuses on several specific types of enthalpy changes, all measured under standard conditions (25°C, 1 atmosphere pressure).
1. Standard Enthalpy of Combustion (ΔHc°)
The enthalpy change when one mole of a substance is completely burned in oxygen under standard conditions.
Example:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l) ΔHc° = -890 kJ/mol
This means burning one mole of methane releases 890 kJ of energy.
2. Standard Enthalpy of Formation (ΔHf°)
The enthalpy change when one mole of a compound is formed from its elements in their standard states.
Example:
C(s) + O₂(g) → CO₂(g) ΔHf° = -394 kJ/mol
Important: The standard enthalpy of formation of any element in its standard state is zero.
3. Standard Enthalpy of Neutralization (ΔHn°)
The enthalpy change when one mole of water is formed from the neutralization of an acid and a base under standard conditions.
Example:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) ΔHn° = -57 kJ/mol
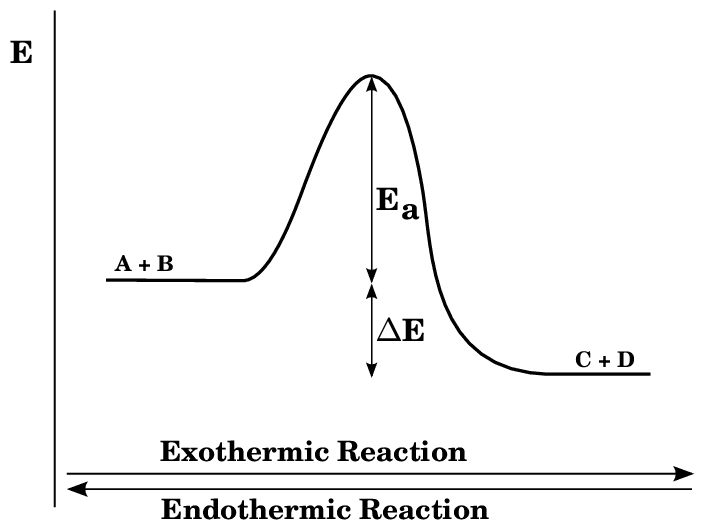
Energy Level Diagrams: Your Visual Guide
Energy level diagrams are like roadmaps for chemical reactions, showing the energy journey from reactants to products.
Components of Energy Level Diagrams:
- Y-axis: Energy (usually in kJ/mol)
- X-axis: Reaction progress/time
- Starting level: Energy of reactants
- Ending level: Energy of products
- Activation energy: The energy barrier that must be overcome
Activation Energy (Ea)
Think of activation energy as the “energy hill” that reactants must climb before they can transform into products. It’s like needing to push a boulder up a hill before it can roll down the other side.
Key Points:
- All reactions need activation energy
- Catalysts lower activation energy but don’t change ΔH
- Higher Ea = slower reaction rate
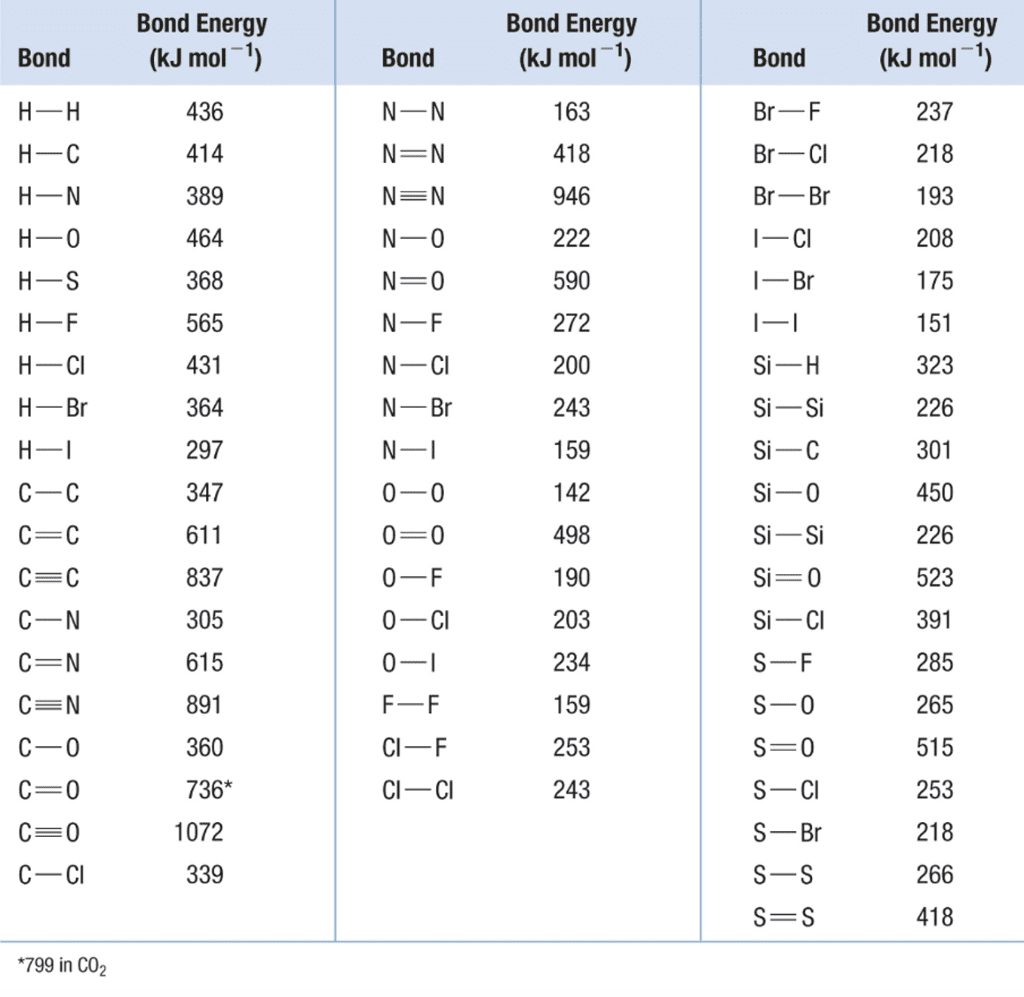
Bond Energy: The Energy Accountant’s Approach
Understanding bond energies helps us calculate enthalpy changes and understand why reactions are exothermic or endothermic.
What Is Bond Energy?
Bond energy is the energy required to break one mole of a particular bond in gaseous molecules, or the energy released when one mole of bonds is formed.
Key Principle:
- Breaking bonds requires energy (endothermic process)
- Making bonds releases energy (exothermic process)
Calculating ΔH Using Bond Energies
Formula: ΔH = Energy required to break bonds – Energy released when bonds form
Step-by-step approach:
- Identify all bonds broken in reactants
- Calculate total energy required to break these bonds
- Identify all bonds formed in products
- Calculate total energy released when forming these bonds
- Apply the formula
Worked Example:
Calculate ΔH for: H₂(g) + Cl₂(g) → 2HCl(g)
Given bond energies:
- H-H: 436 kJ/mol
- Cl-Cl: 243 kJ/mol
- H-Cl: 431 kJ/mol
Solution:
Energy required to break bonds = 436 + 243 = 679 kJ/mol
Energy released making bonds = 2 × 431 = 862 kJ/mol
ΔH = 679 – 862 = -183 kJ/mol (exothermic)
Hess’s Law: The Energy Conservation Detective
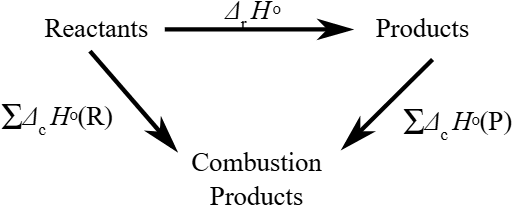
Hess’s Law states that the total enthalpy change for a reaction is independent of the route taken, provided the initial and final conditions are the same.
Why Is Hess’s Law Useful?
Sometimes we can’t measure the enthalpy change of a reaction directly, but we can calculate it using known enthalpy changes of related reactions.
Think of it like this: Whether you climb a mountain via a steep direct path or take several gentler zigzag paths, the total height gained is the same.
Using Hess’s Law with Formation Enthalpies
Formula: ΔH°reaction = Σ ΔHf°(products) – Σ ΔHf°(reactants)
Worked Example:
Calculate ΔH° for: 2NO₂(g) → N₂O₄(g)
Given: ΔHf°(NO₂) = +33 kJ/mol, ΔHf°(N₂O₄) = +10 kJ/mol
Solution:
ΔH° = [1 × 10] – [2 × 33] = 10 – 66 = -56 kJ/mol
Practical Applications: Where You’ll See This in Real Life
1. Fuel Efficiency
Understanding combustion enthalpy helps engineers design more efficient engines and choose better fuels. Hydrogen has a high enthalpy of combustion (-286 kJ/mol), making it an excellent clean fuel.
2. Food and Metabolism
Your body breaks down glucose through respiration:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O ΔH = -2800 kJ/mol
This energy powers everything you do!
3. Industrial Processes
The Haber process for making ammonia:
N₂ + 3H₂ ⇌ 2NH₃ ΔH = -92 kJ/mol
The exothermic nature helps maintain reaction temperature.
4. Hand Warmers and Cold Packs
- Hand warmers use exothermic reactions (iron oxidation)
- Cold packs use endothermic processes (ammonium nitrate dissolving)
Common Exam Questions and How to Tackle Them
Question 1: Energy Level Diagrams
Typical question: “Draw an energy level diagram for an exothermic reaction and label the activation energy and enthalpy change.”
Approach:
- Draw and label axes
- Mark reactants level (higher for exothermic)
- Mark products level (lower for exothermic)
- Draw activation energy peak
- Mark ΔH with downward arrow
Question 2: Bond Energy Calculations
Typical question: “Calculate the enthalpy change for a reaction using bond energies.”
Approach:
- List all bonds broken and formed
- Calculate energy for breaking bonds (positive)
- Calculate energy for forming bonds (negative)
- Apply: ΔH = bonds broken – bonds formed
Question 3: Hess’s Law Problems
Typical question: “Use the given formation enthalpies to calculate the enthalpy of reaction.”
Approach:
- Write the balanced equation
- Apply: ΔH = Σ ΔHf°(products) – Σ ΔHf°(reactants)
- Don’t forget coefficients in the balanced equation
Common Mistakes to Avoid
1. Sign Errors
- Mistake: Confusing positive and negative values
- Solution: Remember exothermic = negative ΔH, endothermic = positive ΔH
2. Units Confusion
- Mistake: Mixing kJ and kJ/mol
- Solution: Always check if the value is per mole or total
3. Bond Energy Direction
- Mistake: Adding when you should subtract in bond energy calculations
- Solution: Remember the formula: ΔH = Energy to break bonds – Energy released making bonds
4. Hess’s Law Setup
- Mistake: Wrong signs when manipulating equations
- Solution: If you reverse an equation, change the sign of ΔH
Key Formulas and Equations Box
Essential Formulas:
- ΔH = H(products) – H(reactants)
- ΔH = Energy required to break bonds – Energy released making bonds
- ΔH°reaction = Σ ΔHf°(products) – Σ ΔHf°(reactants)
- For combustion: Fuel + O₂ → CO₂ + H₂O
- For neutralization: Acid + Base → Salt + Water
Important Constants:
- Standard conditions: 25°C (298K), 1 atm
- ΔHn° for strong acid + strong base ≈ -57 kJ/mol
Quick Revision Notes
Energy Changes:
- Exothermic: ΔH negative, energy released, surroundings get warmer
- Endothermic: ΔH positive, energy absorbed, surroundings get cooler
Enthalpy Types:
- Formation (ΔHf°): Elements → 1 mole compound
- Combustion (ΔHc°): 1 mole substance + O₂ → oxides
- Neutralization (ΔHn°): Acid + base → 1 mole water
Bond Energy Rules:
- Breaking bonds = endothermic (requires energy)
- Making bonds = exothermic (releases energy)
- Net result determines if reaction is exothermic or endothermic
Hess’s Law:
- Total enthalpy change is independent of pathway
- Use when direct measurement isn’t possible
- ΔH for forward reaction = -ΔH for reverse reaction
Test Yourself: Practice Questions
- Energy Level Diagram: Draw and label an energy level diagram for the endothermic reaction: CaCO₃(s) → CaO(s) + CO₂(g)
- Bond Energy Calculation: Calculate ΔH for: CH₄ + 2O₂ → CO₂ + 2H₂O
Given: C-H = 413 kJ/mol, O=O = 498 kJ/mol, C=O = 805 kJ/mol, O-H = 464 kJ/mol - Hess’s Law: If ΔHf°(CO₂) = -394 kJ/mol and ΔHf°(H₂O) = -286 kJ/mol, calculate ΔHc° for hydrogen.
- Identification: Classify these as exothermic or endothermic:
- Photosynthesis
- Respiration
- Melting ice
- Condensing steam
Study Tips for Exam Success
1. Practice Energy Level Diagrams
Draw them regularly until you can sketch them quickly and accurately. Remember to always label axes and show the correct energy relationships.
2. Master the Math
Chemical energetics involves calculations. Practice bond energy problems and Hess’s Law calculations until they become automatic.
3. Understand the Concepts
Don’t just memorize – understand why reactions are exothermic or endothermic in terms of bond breaking and forming.
4. Use Real Examples
Connect abstract concepts to real-world examples. This helps with understanding and memory retention.
5. Check Your Signs
Always double-check whether your ΔH values should be positive or negative. This is where many students lose marks.
Looking Ahead: Your Next Steps
Congratulations! You’ve now covered the fundamental concepts of chemical energetics. This knowledge forms the foundation for understanding:
- Reaction rates and catalysis (how energy barriers affect reaction speed)
- Chemical equilibrium (how energy changes affect reaction position)
- Organic chemistry (energy changes in organic reactions)
- Industrial chemistry (optimizing processes using energy considerations)
Related Topics to Explore:
- Topic 8: Rates of Reaction (how activation energy affects reaction speed)
- Topic 9: The Periodic Table (trends in bond energies across periods)
- Topic 14: Organic Chemistry (energy changes in organic reactions)
Final Motivation
Remember, chemical energetics isn’t just about memorizing definitions and formulas – it’s about understanding the fundamental driving forces behind all chemical changes. Every reaction you’ll study in chemistry, from the simple burning of methane to the complex biochemical processes in your body, follows these same energy principles.
Take your time with this topic, practice regularly, and don’t hesitate to revisit concepts that challenge you. With persistence and the right approach, you’ll master chemical energetics and be well-prepared for your IGCSE Chemistry exam.
Recommended –


2 thoughts on “IGCSE (Cambridge) Chemistry Topic 5: Chemical Energetics | Your Complete Study Guide”