Why Environmental Chemistry Matters More Than Ever
Picture this: You wake up, check the air quality index on your phone, drink filtered water, and worry about climate change – all before breakfast! Welcome to the world where chemistry meets our environment, and where your IGCSE Chemistry knowledge becomes incredibly relevant to real life.
Environmental chemistry isn’t just another topic to memorize for your Cambridge Chemistry 0620 exam. It’s the science behind every environmental news headline, every sustainability initiative, and every solution to our planet’s biggest challenges. Whether you’re passionate about saving the planet or simply want to ace your exams, this comprehensive guide will transform you from confused to confident in Topic 10.
Don’t worry if chemistry feels overwhelming sometimes – we’ve all been there! This guide breaks down complex environmental processes into bite-sized, understandable pieces. By the end, you’ll not only understand how human activities affect our environment but also feel prepared to tackle any exam question that comes your way.
What You’ll Learn in IGCSE Chemistry Topic 10
The Chemistry of the Environment covers four main areas that are essential for both your exams and understanding our world:
• Air Quality and Pollution – Understanding what we breathe and how it affects us • Water Systems – From natural water cycles to water treatment processes
• Environmental Impact of Human Activities – How our actions change the planet’s chemistry • Solutions and Sustainability – Chemical approaches to environmental problems
Each section builds upon your previous chemistry knowledge, so don’t panic if some concepts seem challenging at first. We’ll connect everything to what you already know about atoms, molecules, and chemical reactions.
1: Air Quality and Atmospheric Chemistry
Understanding Our Atmosphere: The Invisible Ocean Above Us
Think of Earth’s atmosphere as a massive chemical reactor that never stops working. The air you breathe is a carefully balanced mixture of gases, and even tiny changes can have huge consequences.
Normal Atmospheric Composition:
- Nitrogen (N₂): 78%
- Oxygen (O₂): 21%
- Argon (Ar): 0.9%
- Carbon dioxide (CO₂): 0.04% (and rising!)
- Water vapor (H₂O): Variable (0-4%)
- Trace gases: Less than 0.1%
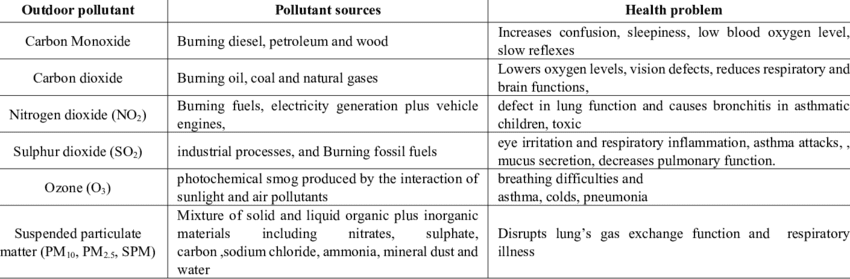
Air Pollutants: The Unwanted Guests
Air pollution occurs when harmful substances enter our atmosphere in concentrations that damage health or the environment. Let’s explore the major culprits:
Primary Pollutants (Released Directly):
- Carbon Monoxide (CO)
- Source: Incomplete combustion of carbon-containing fuels
- Chemical equation: 2C + O₂ → 2CO (when oxygen is limited)
- Effects: Prevents oxygen transport in blood; extremely dangerous in enclosed spaces
- Real-life example: Car exhaust in poorly ventilated garages
- Sulfur Dioxide (SO₂)
- Source: Burning fossil fuels containing sulfur compounds
- Chemical equation: S + O₂ → SO₂
- Effects: Respiratory problems, contributes to acid rain
- Memory tip: “Sulfur Dioxide = Sour rain”
- Nitrogen Oxides (NOₓ)
- Source: High-temperature combustion in engines and power plants
- Chemical equation: N₂ + O₂ → 2NO (at high temperatures)
- Effects: Respiratory irritation, contributes to smog and acid rain
- Particulate Matter (PM)
- Source: Combustion, industrial processes, natural sources
- Effects: Lung damage, cardiovascular problems
- Size matters: PM2.5 (particles smaller than 2.5 micrometers) are most dangerous
Secondary Pollutants (Formed in the Atmosphere):
- Ground-level Ozone (O₃)
- Formation: NOₓ + Volatile Organic Compounds (VOCs) + Sunlight → O₃
- Effects: Respiratory problems, crop damage
- Important distinction: Good ozone (stratosphere) vs. bad ozone (ground level)
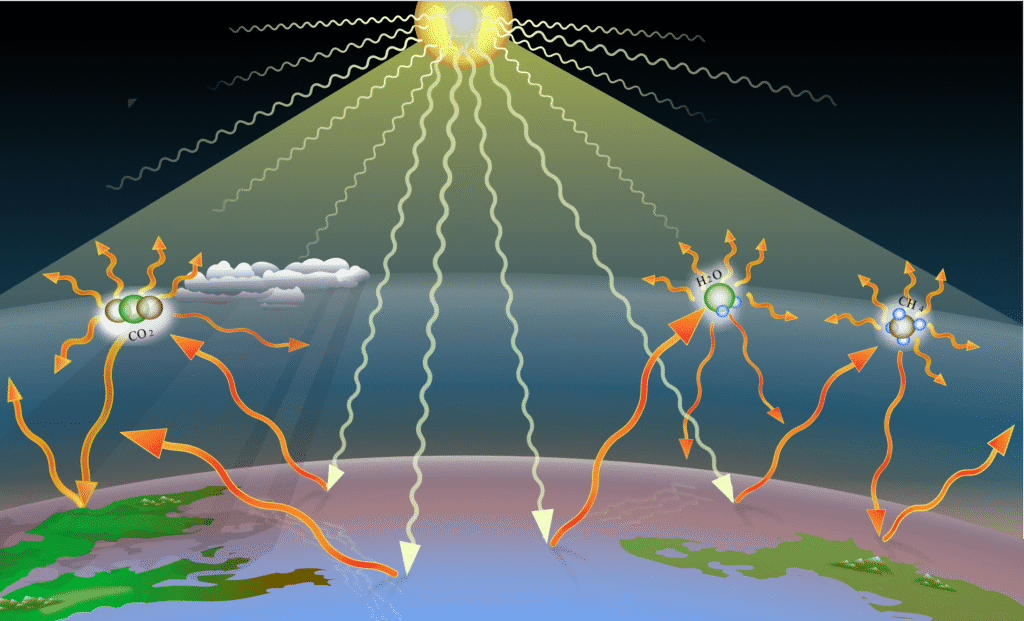
The Greenhouse Effect: Earth’s Natural Blanket
The greenhouse effect is like Earth wearing a blanket – it keeps us warm, but too thick a blanket makes us overheat!
How It Works:
- Solar radiation reaches Earth’s surface
- Earth’s surface absorbs energy and warms up
- Earth radiates heat back as infrared radiation
- Greenhouse gases absorb and re-emit this infrared radiation
- Some heat is trapped, keeping Earth warmer than it would be otherwise
Natural Greenhouse Gases:
- Water vapor (H₂O): Most abundant, but concentration varies naturally
- Carbon dioxide (CO₂): Major long-lived greenhouse gas
- Methane (CH₄): Much more potent than CO₂ but less abundant
- Nitrous oxide (N₂O): From natural soil processes and human activities
Enhanced Greenhouse Effect: Human activities increase greenhouse gas concentrations, leading to global warming:
- Burning fossil fuels increases CO₂
- Agriculture and landfills increase CH₄
- Industrial processes increase N₂O and synthetic gases
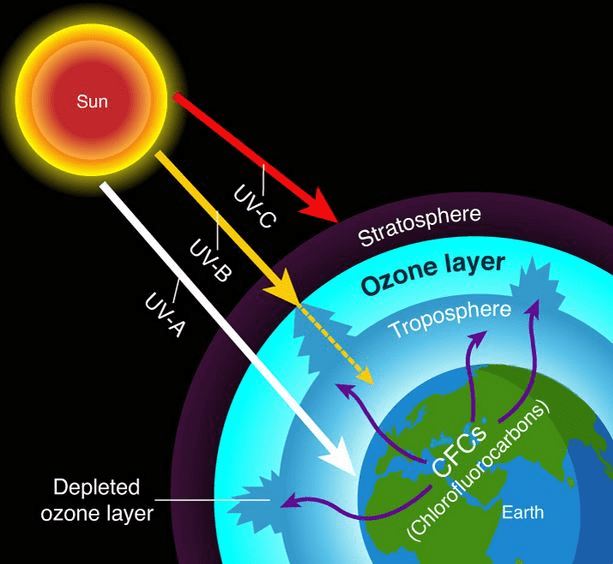
Ozone Layer Depletion: Our Protective Shield Under Attack
The ozone layer in the stratosphere protects us from harmful UV radiation. Think of it as Earth’s natural sunscreen!
Ozone Formation and Destruction (Natural Cycle): Formation: O₂ + UV light → 2O (oxygen atoms) O + O₂ → O₃ (ozone)
Destruction: O₃ + UV light → O₂ + O O + O₃ → 2O₂
Human Impact – CFCs (Chlorofluorocarbons): CFCs were once common in refrigerators and aerosols. In the stratosphere:
- UV light breaks down CFCs, releasing chlorine atoms
- Cl + O₃ → ClO + O₂
- ClO + O → Cl + O₂
- The chlorine atom is regenerated and can destroy more ozone
One chlorine atom can destroy thousands of ozone molecules – that’s why CFCs are so dangerous!
Montreal Protocol Success Story: The 1987 Montreal Protocol banned CFCs globally, showing that international cooperation can solve environmental problems. The ozone hole is slowly recovering!
2: Water Chemistry and Treatment
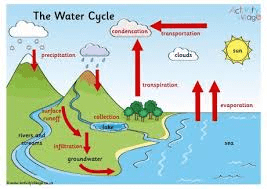
The Water Cycle: Nature’s Recycling System
Water continuously moves through the environment in a process powered by solar energy. Understanding this cycle is crucial for appreciating water quality issues.
Key Processes:
- Evaporation: H₂O(l) → H₂O(g) – Solar energy converts liquid water to vapor
- Transpiration: Plants release water vapor through leaves
- Condensation: H₂O(g) → H₂O(l) – Water vapor forms droplets in clouds
- Precipitation: Rain, snow, or hail returns water to Earth’s surface
Water Pollution: Contaminating Our Most Precious Resource
Clean water is essential for life, but human activities introduce various pollutants that make water unsafe for consumption or harmful to aquatic ecosystems.
Types of Water Pollutants:
- Chemical Pollutants
- Heavy metals (lead, mercury, cadmium): Toxic even in small amounts
- Pesticides and herbicides: Can persist in the environment
- Industrial chemicals: May be carcinogenic or toxic
- Oil spills: Create films that prevent oxygen dissolution
- Biological Pollutants
- Bacteria and viruses: Cause waterborne diseases
- Excess nutrients (eutrophication): Lead to algae blooms and oxygen depletion
- Physical Pollutants
- Suspended solids: Make water cloudy and can carry other pollutants
- Thermal pollution: Hot water discharge affects aquatic life
- Plastic waste: Physical hazard and source of microplastics
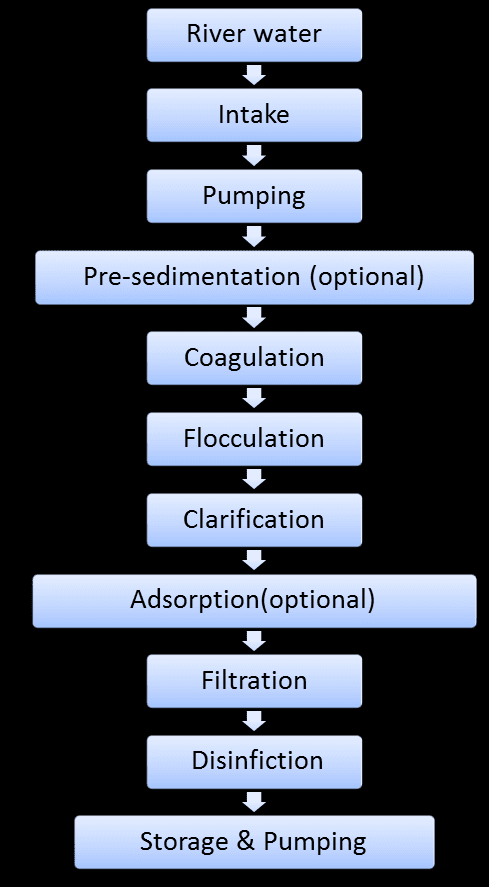
Water Treatment: Making Water Safe to Drink
Water treatment removes pollutants to make water safe for human consumption. The process involves several stages:
Stage 1: Screening and Pre-treatment
- Large debris removal using screens and filters
- Addition of coagulants like aluminum sulfate [Al₂(SO₄)₃]
Stage 2: Coagulation and Flocculation
- Coagulants neutralize charges on particles
- Small particles clump together to form larger flocs
- Chemical equation: Al₂(SO₄)₃ + 6H₂O → 2Al(OH)₃ + 3H₂SO₄
Stage 3: Sedimentation
- Gravity settles flocs to the bottom of settling tanks
- Clear water rises to the top
Stage 4: Filtration
- Water passes through sand and gravel filters
- Removes remaining suspended particles and some microorganisms
Stage 5: Disinfection
- Chlorine addition kills remaining bacteria and viruses
- Chemical equation: Cl₂ + H₂O → HCl + HClO (hypochlorous acid)
- HClO is the active disinfectant
Stage 6: pH Adjustment
- Addition of lime [Ca(OH)₂] to optimize pH for distribution
Alternative Water Treatment Methods
- Reverse Osmosis
- Forces water through semi-permeable membranes
- Removes dissolved salts and many contaminants
- Used for desalination and producing ultra-pure water
- UV Disinfection
- UV light damages DNA in microorganisms
- No chemical addition required
- Effective against chlorine-resistant pathogens
- Activated Carbon Filtration
- Adsorbs organic compounds and chlorine
- Improves taste and odor
- Used in home water filters
3: Acid Rain – When the Sky Turns Sour
Formation of Acid Rain
Normal rainwater is slightly acidic (pH ≈ 5.6) due to dissolved carbon dioxide: CO₂ + H₂O → H₂CO₃ (carbonic acid)
Acid rain forms when sulfur dioxide and nitrogen oxides dissolve in atmospheric water:
Sulfuric Acid Formation:
- SO₂ + ½O₂ → SO₃ (in the atmosphere)
- SO₃ + H₂O → H₂SO₄ (sulfuric acid)
Nitric Acid Formation:
- 2NO + O₂ → 2NO₂
- 3NO₂ + H₂O → 2HNO₃ + NO (nitric acid)
Acid rain typically has a pH between 4.0-4.5, sometimes as low as 2.0 in heavily polluted areas!
Effects of Acid Rain
Environmental Impact:
- Forest damage: Acid rain leaches nutrients from soil and damages tree leaves
- Aquatic ecosystem disruption: Lower pH kills fish and other aquatic organisms
- Soil acidification: Reduces agricultural productivity
Built Environment:
- Stone and metal corrosion: Particularly damaging to limestone and marble
- CaCO₃ + H₂SO₄ → CaSO₄ + H₂O + CO₂ (limestone dissolution)
- Infrastructure damage: Bridges, buildings, and monuments deteriorate faster
Controlling Acid Rain
Source Reduction:
- Use low-sulfur fuels
- Install scrubbers in power plants: CaO + SO₂ → CaSO₃ (calcium sulfite)
- Catalytic converters in vehicles reduce NOₓ emissions
Environmental Remediation:
- Liming of acidified lakes: CaO + 2H⁺ → Ca²⁺ + H₂O
- Forest management to restore damaged ecosystems
4: Solutions and Sustainability
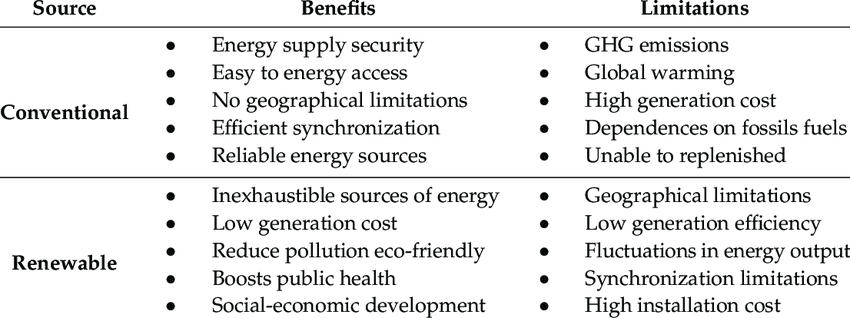
Green Chemistry Principles
Green chemistry focuses on designing chemical processes that reduce environmental impact:
- Prevention: Better to prevent waste than clean it up
- Atom Economy: Maximize incorporation of reactants into final products
- Less Hazardous Synthesis: Use safer chemicals and conditions
- Renewable Feedstocks: Use renewable raw materials when possible
- Energy Efficiency: Conduct reactions at room temperature and pressure when possible
Alternative Energy and Chemistry
Solar Energy:
- Photovoltaic cells convert sunlight directly to electricity
- Uses semiconductor materials like silicon
Fuel Cells:
- Convert chemical energy directly to electrical energy
- Hydrogen fuel cell: 2H₂ + O₂ → 2H₂O + electrical energy
- Only byproduct is water!
Biofuels:
- Ethanol from fermentation: C₆H₁₂O₆ → 2C₂H₅OH + 2CO₂
- Biodiesel from vegetable oils
- Carbon neutral if sustainably produced
Key Chemical Equations and Formulas
**Combustion and Pollution:**
Complete combustion: CH₄ + 2O₂ → CO₂ + 2H₂O
Incomplete combustion: 2CH₄ + 3O₂ → 2CO + 4H₂O
Sulfur dioxide formation: S + O₂ → SO₂
Nitrogen oxide formation: N₂ + O₂ → 2NO
**Acid Rain Formation:**
SO₂ + ½O₂ → SO₃
SO₃ + H₂O → H₂SO₄
3NO₂ + H₂O → 2HNO₃ + NO
**Ozone Chemistry:**
Ozone formation: O₂ + UV → 2O; O + O₂ → O₃
CFC ozone destruction: Cl + O₃ → ClO + O₂
**Water Treatment:**
Coagulation: Al₂(SO₄)₃ + 6H₂O → 2Al(OH)₃ + 3H₂SO₄
Disinfection: Cl₂ + H₂O → HCl + HClO
**Neutralization:**
Limestone with acid rain: CaCO₃ + H₂SO₄ → CaSO₄ + H₂O + CO₂
Lake liming: CaO + 2H⁺ → Ca²⁺ + H₂O
Quick Revision Notes
Air Pollution Quick Facts:
• CO prevents oxygen transport – deadly in enclosed spaces • SO₂ causes acid rain – remember “Sulfur = Sour rain” • NOₓ forms from high-temperature combustion • Ground-level ozone = bad; stratospheric ozone = good • CFCs destroy ozone through chlorine radical chain reactions
Water Treatment Steps (Remember: “Screen, Coagulate, Settle, Filter, Disinfect”):
- Screen – Remove large debris
- Coagulate – Add Al₂(SO₄)₃ to clump particles
- Settle – Let flocs sink in sedimentation tanks
- Filter – Pass through sand/gravel filters
- Disinfect – Add chlorine to kill microorganisms
Acid Rain Memory Tricks:
• Normal rain pH ≈ 5.6 (slightly acidic from CO₂) • Acid rain pH < 4.5 (from SO₂ and NOₓ) • Limestone buildings suffer most: CaCO₃ + acid → dissolves away
Environmental Solutions:
• Montreal Protocol success – CFCs banned, ozone layer recovering • Catalytic converters reduce NOₓ from cars • Scrubbers remove SO₂ from power plants • Green chemistry – design processes to prevent pollution
Common Exam Questions and How to Tackle Them
Question 1: Explaining Pollution Formation
“Explain how acid rain forms and state one environmental effect.”
Answer Structure:
- State the pollutants involved (SO₂, NOₓ)
- Give sources of these pollutants
- Write chemical equations for acid formation
- State a specific environmental effect with explanation
Question 2: Water Treatment Process
“Describe the stages involved in water treatment.”
Answer Structure:
- List stages in order
- Explain purpose of each stage
- Include relevant chemical equations
- Mention final quality checks
Question 3: Comparing Solutions
“Compare the advantages and disadvantages of different methods to reduce air pollution.”
Answer Structure:
- Identify different methods (catalytic converters, scrubbers, alternative fuels)
- State advantages of each
- State disadvantages of each
- Make a conclusion about effectiveness
Question 4: Data Analysis
Questions involving pH measurements, pollution levels, or treatment efficiency
Answer Structure:
- Read data carefully from tables/graphs
- Identify trends or patterns
- Explain observations using chemical knowledge
- Make predictions or conclusions
Test Yourself: Practice Questions
- Knowledge Check: What is the difference between primary and secondary air pollutants? Give one example of each.
- Application: A lake has become acidified due to acid rain. Suggest two methods to restore the lake’s pH and explain how each method works chemically.
- Analysis: The concentration of CO₂ in the atmosphere has increased from 315 ppm in 1960 to 410 ppm today. Explain how this change affects the greenhouse effect and suggest two ways to reduce CO₂ emissions.
- Evaluation: Compare the effectiveness of chlorine and UV light for water disinfection. Include advantages and disadvantages of each method.
- Synthesis: Design a plan to reduce air pollution in a city. Your plan should address at least three different types of pollutants and explain the chemistry behind each solution.
Common Mistakes to Avoid
Confusing good and bad ozone – Remember: stratospheric ozone protects us, ground-level ozone harms us
Mixing up greenhouse effect and ozone depletion – These are separate environmental issues with different causes
Forgetting to balance chemical equations – Always check your equations are balanced
Not explaining the chemistry – Don’t just state facts; explain the chemical processes involved
Ignoring units and significant figures – Pay attention to pH scales, concentrations, and measurements
Writing generic answers – Be specific about pollutants, effects, and solutions
Memory Tips and Mnemonics
“SCOOP” for greenhouse gases: Sulfur hexafluoride, Carbon dioxide, Oxygen (ozone), Other halocarbons, Plus methane
“SCFCD” for water treatment: Screen, Coagulate, Flocculate, Clarify, Disinfect
“SOx = Sour rain” – Sulfur oxides make acid rain
“NO way for NOx” – Nitrogen oxides are bad for air quality
“pH scale memory: “Pure water = 7, rain = 5.6, acid rain = 4 and below”
Connecting Chemistry to Current Events
Environmental chemistry isn’t just textbook knowledge – it’s happening all around us! Here are ways to connect your learning to real-world events:
• Climate Change News: When you hear about CO₂ levels or temperature records, you understand the greenhouse effect chemistry behind it
• Air Quality Alerts: Those pollution warnings on your weather app relate directly to the pollutants you’ve studied
• Water Crises: Understanding water treatment helps you appreciate clean water access issues globally
• Environmental Policies: The Montreal Protocol success story shows how chemistry knowledge leads to environmental solutions
• Renewable Energy: Solar panels, fuel cells, and biofuels all involve chemistry principles you’re learning
Your Next Steps to Exam Success
Congratulations! You’ve just completed a comprehensive journey through IGCSE Chemistry Topic 10. Here’s your roadmap to exam excellence:
Exam Technique Reminders:
• Read questions carefully – they often ask for specific numbers of points • Use chemical equations to support your explanations • Draw clear, labeled diagrams when asked • Show your working in calculation questions • Manage your time – don’t spend too long on any single question
Beyond the Exam:
Environmental chemistry knowledge opens doors to many exciting careers:
- Environmental consultant
- Water treatment engineer
- Air quality specialist
- Green chemistry researcher
- Sustainability manager
- Environmental policy advisor
Remember, every environmental problem is also a chemistry opportunity for solution!
Related Topics to Explore
Ready to expand your chemistry knowledge? These related topics will deepen your understanding:
• Topic 8: Acids, Bases and Salts – Essential for understanding acid rain and water treatment • Topic 9: The Periodic Table – Helps explain why certain elements are environmental concerns • Topic 11: Petrochemicals and Polymers – Connects to plastic pollution and alternative materials • Topic 12: Chemical Calculations – Practice calculating pollution concentrations and treatment efficiency
Final Encouragement
Environmental chemistry might seem challenging, but remember – you’re studying the science that will help solve our planet’s biggest challenges. Every concept you master brings you closer to understanding how chemistry can create a more sustainable future.
Don’t get discouraged if some topics feel difficult at first. Chemistry is like learning a new language, and with practice, those chemical equations and environmental processes will become second nature. You have all the tools you need to succeed – now it’s time to put them to work!
Your IGCSE Chemistry 0620 exam is just one step in your journey. The knowledge you’re gaining today will serve you well whether you continue with A-level Chemistry, pursue environmental science, or simply become a more informed global citizen.
Keep studying, stay curious, and remember – the future of our environment depends on people who understand the chemistry behind it. That includes you!
Good luck with your studies, and remember – you’ve got this!
Recommended –

1 thought on “GCSE (Cambridge) Chemistry Topic 10: Chemistry of the Environment | Your Complete Study Guide”