Welcome to the Fascinating World of Organic Chemistry
Picture this: you’re sipping a cup of coffee, wearing a polyester shirt, and scrolling through your phone with a plastic case. What do all these everyday items have in common? They’re all made from organic compounds! From the caffeine that keeps you alert to the synthetic materials surrounding you, organic chemistry is literally everywhere in your daily life.
If you’ve been dreading IGCSE Chemistry Topic 11, thinking it’s all about memorizing countless formulas and structures, let me change that perspective right now. Organic chemistry is actually one of the most logical and pattern-based topics in your entire chemistry syllabus. Once you understand the fundamental principles, everything else falls into place like pieces of a perfectly crafted puzzle.
In this comprehensive guide, we’ll transform what might seem like an overwhelming topic into manageable, understandable chunks. By the end of this post, you’ll not only master the content for your IGCSE Chemistry 0620 exam but also develop a genuine appreciation for how organic chemistry shapes our modern world.
What Exactly is Organic Chemistry? Breaking Down the Basics
The Simple Definition That Changes Everything
Organic chemistry is the study of carbon-containing compounds. But here’s the fascinating part – carbon is like the ultimate team player in the periodic table. It can form four bonds with other atoms, creating an incredible variety of structures that form the backbone of life itself.
Think of carbon as the LEGO brick of the chemical world. Just as you can build countless structures with LEGO bricks, carbon atoms can connect in endless ways to create everything from the simplest methane molecule to complex proteins in your body.
Why Carbon is So Special
Carbon’s unique properties make it perfect for forming organic compounds:
- Tetravalency: Forms exactly four bonds
- Catenation: Can bond with other carbon atoms to form chains
- Versatile bonding: Can form single, double, or triple bonds
- Stable compounds: Creates molecules that don’t fall apart easily
The Foundation: Understanding Hydrocarbons
Alkanes – The Saturated Superstars
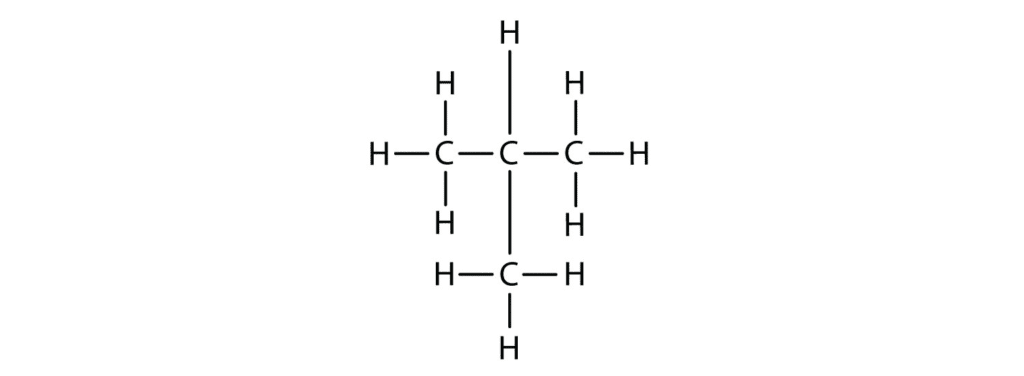
Alkanes are the simplest organic compounds, containing only carbon and hydrogen atoms connected by single bonds. They’re called “saturated” because they’re completely filled with hydrogen atoms – think of them as carbon chains wearing hydrogen sweaters!
The Alkane Family Tree:
- Methane (CH₄) – 1 carbon atom
- Ethane (C₂H₆) – 2 carbon atoms
- Propane (C₃H₈) – 3 carbon atoms
- Butane (C₄H₁₀) – 4 carbon atoms
Memory Tip: Remember “My Elephant Produces Butter” for Methane, Ethane, Propane, Butane!
General Formula: CₙH₂ₙ₊₂
Properties of Alkanes:
- Generally unreactive (hence called “paraffins” meaning “little affinity”)
- Used as fuels (natural gas, petroleum)
- Boiling points increase with molecular size
- Insoluble in water but soluble in organic solvents
Alkenes – The Reactive Rebels
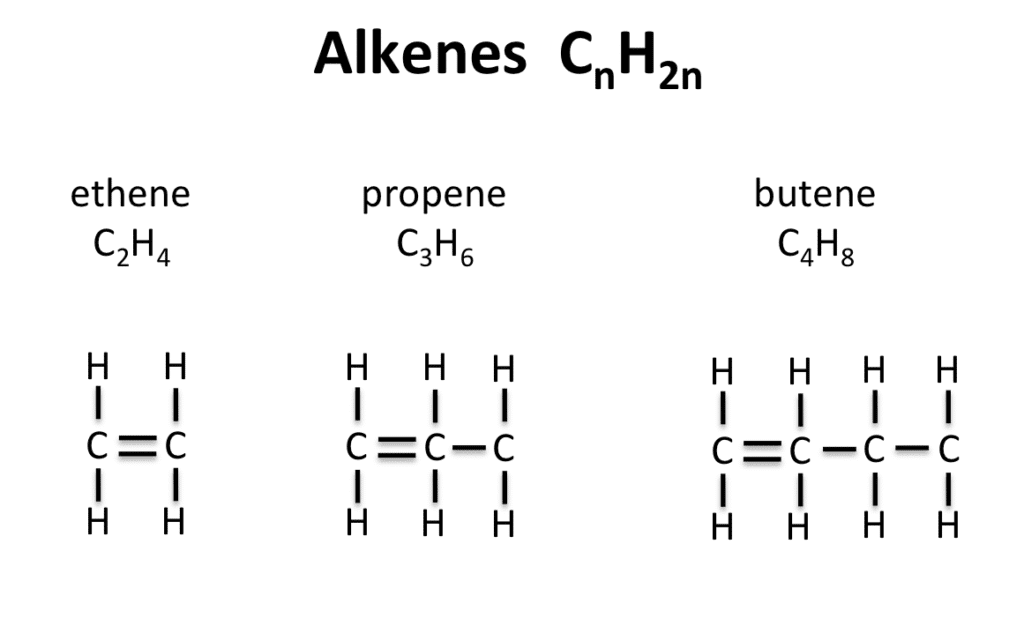
Alkenes contain at least one double bond between carbon atoms, making them much more reactive than alkanes. The double bond is like a spring under tension – it’s ready to react!
The Alkene Family:
- Ethene (C₂H₄) – simplest alkene
- Propene (C₃H₆) – three carbon atoms
- Butene (C₄H₈) – four carbon atoms
General Formula: CₙH₂ₙ
Key Reactions of Alkenes:
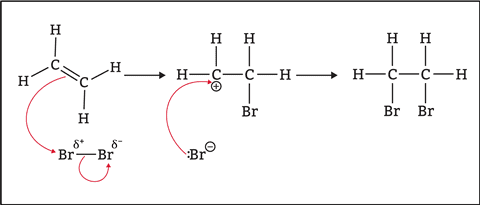
1. Addition Reactions
Alkenes readily undergo addition reactions where the double bond opens up to accommodate new atoms:
- Hydrogenation: Alkene + H₂ → Alkane
- Bromination: Alkene + Br₂ → Dibromoalkane
- Hydration: Alkene + H₂O → Alcohol
Test for Unsaturation: Bromine water test
- Alkenes turn orange bromine water colorless
- Alkanes show no change with bromine water
Functional Groups – The Chemistry Game Changers
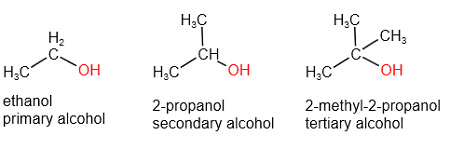
Alcohols – The Social Molecules
Alcohols contain the hydroxyl group (-OH) attached to a carbon atom. They’re like alkanes that have learned to be social – the -OH group makes them much more interactive with other molecules.
Primary Alcohols (OH group at the end):
- Methanol (CH₃OH) – wood alcohol
- Ethanol (C₂H₅OH) – drinking alcohol
Secondary Alcohols (OH group in the middle):
- 2-propanol (isopropanol) – rubbing alcohol
Properties of Alcohols:
- Higher boiling points than corresponding alkanes (due to hydrogen bonding)
- Soluble in water (small alcohols)
- Can be oxidized to form other compounds
Important Reactions of Alcohols:
1. Combustion:
C₂H₅OH + 3O₂ → 2CO₂ + 3H₂O
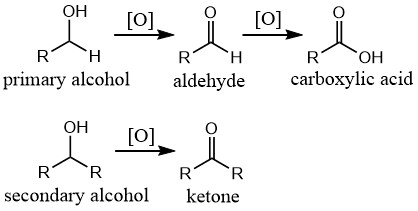
2. Oxidation:
- Primary alcohols → Aldehydes → Carboxylic acids
- Secondary alcohols → Ketones
- Tertiary alcohols → No reaction under mild conditions
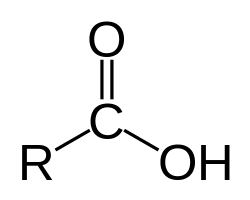
Carboxylic Acids – The Sour Specialists
Carboxylic acids contain the carboxyl group (-COOH) and are responsible for the sour taste in vinegar, citrus fruits, and many other foods.
Common Examples:
- Methanoic acid (HCOOH) – formic acid (ant stings!)
- Ethanoic acid (CH₃COOH) – acetic acid (vinegar)
- Propanoic acid (C₂H₅COOH) – food preservative
General Formula: CₙH₂ₙ₊₁COOH
Properties of Carboxylic Acids:
- Weak acids (partially ionize in water)
- Form hydrogen bonds (high boiling points)
- React with metals, bases, and carbonates
- Show typical acid reactions
Key Reactions:
- With metals: 2CH₃COOH + Mg → (CH₃COO)₂Mg + H₂
- With bases: CH₃COOH + NaOH → CH₃COONa + H₂O
- With carbonates: 2CH₃COOH + Na₂CO₃ → 2CH₃COONa + H₂O + CO₂
Polymers – The Giant Molecules
Addition Polymers – Building Big from Small
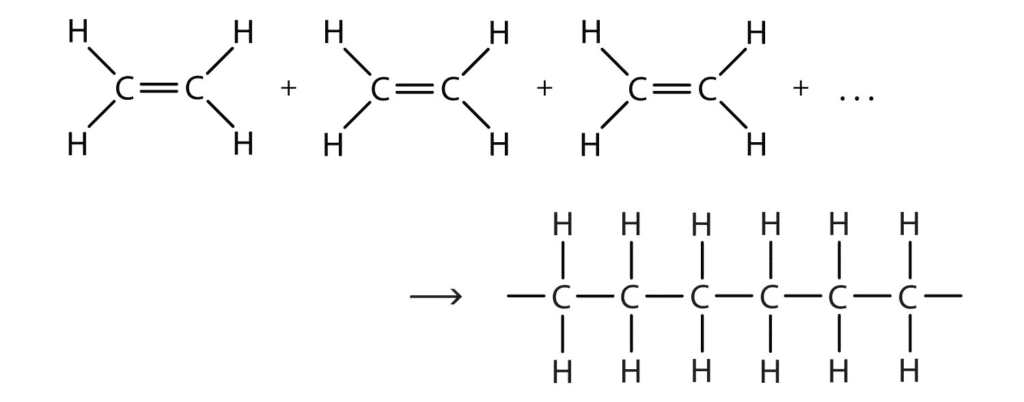
Polymers are giant molecules made by joining thousands of small molecules (monomers) together. It’s like making a chain from paper clips – each paper clip is a monomer, and the entire chain is the polymer.
Polyethene Formation:
nC₂H₄ → (C₂H₄)ₙ
Where ‘n’ can be thousands or even millions!
Common Addition Polymers:
| Monomer | Polymer | Uses |
|---|---|---|
| Ethene | Polyethene | Plastic bags, bottles |
| Propene | Polypropene | Rope, carpets |
| Chloroethene | Polychloroethene (PVC) | Pipes, window frames |
| Styrene | Polystyrene | Packaging, insulation |
Environmental Impact of Polymers
The Good:
- Lightweight and durable
- Prevent food spoilage
- Medical applications
- Energy efficient to produce
The Challenges:
- Non-biodegradable
- Plastic pollution
- Microplastics in ecosystems
Solutions:
- Recycling programs
- Biodegradable alternatives
- Reduce, reuse, recycle approach
Practical Applications – Where Organic Chemistry Meets Real Life
Fuels and Energy
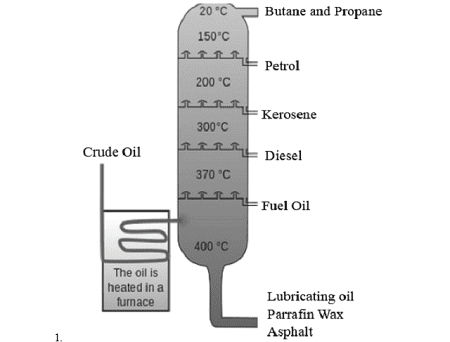
Crude Oil Fractions:
Crude oil is separated by fractional distillation into useful fractions:
- Gases (C₁-C₄): LPG, natural gas
- Petrol (C₅-C₁₀): Car fuel
- Kerosene (C₁₁-C₁₅): Jet fuel
- Diesel (C₁₆-C₂₀): Truck fuel
- Heavy oils (C₂₁+): Lubricants, asphalt
Everyday Organic Compounds
- Ethanol: Alcoholic beverages, fuel additive, antiseptic
- Ethanoic acid: Vinegar, food preservation
- Methane: Natural gas for heating and cooking
- Ethene: Ripening fruit, plastic production
Key Formulas and Equations Box
Essential Formulas:
- Alkanes: CₙH₂ₙ₊₂
- Alkenes: CₙH₂ₙ
- Alcohols: CₙH₂ₙ₊₁OH
- Carboxylic acids: CₙH₂ₙ₊₁COOH
Important Reactions:
- Complete combustion: Hydrocarbon + O₂ → CO₂ + H₂O
- Addition to alkenes: C₂H₄ + Br₂ → C₂H₄Br₂
- Alcohol oxidation: Primary alcohol → Aldehyde → Carboxylic acid
- Esterification: Carboxylic acid + Alcohol → Ester + Water
- Polymerization: nMonomer → Polymer
Quick Revision Notes
Recognition Tests:
- Alkenes: Decolorize orange bromine water
- Alcohols: React with sodium to produce hydrogen gas
- Carboxylic acids: Turn blue litmus red, react with carbonates
Naming Rules:
- Find the longest carbon chain
- Number from the end nearest the functional group
- Name the functional group with its position
- Add prefixes for branches (methyl, ethyl, etc.)
Physical Properties Trends:
- Boiling points increase with molecular size
- Branched molecules have lower boiling points than straight chains
- Functional groups affect solubility and reactivity
Common Mistakes to Avoid
Structure Drawing Errors:
- Forgetting to show all bonds clearly
- Mixing up structural and molecular formulas
- Incorrect placement of functional groups
Naming Mistakes:
- Not finding the longest carbon chain
- Numbering from the wrong end
- Forgetting to indicate position of functional groups
Reaction Confusion:
- Mixing up addition and substitution reactions
- Forgetting conditions needed for reactions
- Incorrect products for oxidation reactions
Test Yourself – Practice Questions
Quick Fire Questions:
- What is the molecular formula of butane?
- Which test distinguishes between alkanes and alkenes?
- What type of alcohol can be oxidized to form a ketone?
- Name the polymer formed from propene.
- What gas is produced when ethanoic acid reacts with calcium carbonate?
Application Questions:
- Explain why alcohols have higher boiling points than alkanes of similar molecular mass.
- Describe how you would distinguish between ethane and ethene using chemical tests.
- Outline the environmental concerns associated with plastic polymers and suggest solutions.
Memory Techniques:
- OILRIG: Oxidation Is Loss (of electrons), Reduction Is Gain
- Functional group family tree: Draw connections between related compounds
- Real-life connections: Link each compound to something you use daily
Your Next Steps to Organic Chemistry Mastery
Congratulations! You’ve just covered one of the most important topics in IGCSE Chemistry. Organic chemistry might have seemed intimidating at first, but now you can see it’s really about understanding patterns and making logical connections.
What to Do Next:
- Practice, Practice, Practice: Work through past paper questions focusing on Topic 11
- Create Summary Sheets: Make one-page summaries for each functional group
- Teach Someone Else: Explaining concepts to others reinforces your own understanding
- Connect to Other Topics: Link organic chemistry to environmental chemistry and industrial processes
Remember:
Every expert was once a beginner. The students who excel in organic chemistry aren’t necessarily the ones who memorize the most – they’re the ones who understand the underlying patterns and can apply them to new situations.
Your IGCSE Chemistry journey is preparing you for exciting possibilities – perhaps a career in medicine, environmental science, materials engineering, or pharmaceutical research. The organic chemistry foundation you’re building now will serve you well in whatever path you choose.
Final Motivation:
Don’t just aim to pass your exam – aim to truly understand and appreciate the elegant logic of organic chemistry. When you can look at a molecule and predict its properties, plan a synthesis pathway, or explain why plastics behave as they do, you’ll have gained something far more valuable than exam marks – you’ll have gained chemical insight that will stay with you for life.
Keep studying, stay curious, and remember – in organic chemistry, every molecule tells a story. Your job is to learn the language to read those stories. You’ve got this!
Recommended –