The Molecular Foundation of Life
Imagine your body as an incredibly complex LEGO structure. Just as LEGO buildings are made from individual bricks, your entire body is constructed from tiny molecular building blocks called biological molecules. Every breath you take, every heartbeat, every thought you think – they all depend on these remarkable chemical compounds working together in perfect harmony.
Welcome to one of the most fascinating topics in IGCSE Biology! Topic 4: Biological Molecules isn’t just about memorizing chemical formulas (though we’ll cover those too). It’s about understanding the very essence of what makes life possible. Whether you’re aiming for that A* grade or simply trying to make sense of complex biochemistry, this comprehensive guide will transform the way you think about the molecules that make you… well, you!
By the end of this journey, you’ll not only understand the four major groups of biological molecules but also appreciate how they work together to create the miracle we call life. Let’s dive in!
What Are Biological Molecules?
Biological molecules, also known as biomolecules, are large complex molecules that are essential for all living organisms. Think of them as life’s toolkit – each type has specific jobs and structures perfectly designed for their functions.
All biological molecules are based on carbon atoms, making them organic compounds. Carbon is special because it can form four strong bonds with other atoms, creating the complex structures needed for life. It’s like having a super-versatile connector piece in our molecular LEGO set!
The four main groups of biological molecules are:
- Carbohydrates – The body’s primary energy source
- Proteins – The workhorses that build and maintain structures
- Lipids – Energy storage and membrane components
- Nucleic Acids – The information storage molecules
Carbohydrates: Life’s Fuel Tank
What Are Carbohydrates?
Carbohydrates are molecules made up of carbon, hydrogen, and oxygen atoms, typically in a 1:2:1 ratio. The name literally means “carbon with water” (carbo + hydrate), which gives you a clue about their composition.
Key Formula to Remember: General formula is (CH₂O)ₙ where n represents the number of carbon atoms.
Types of Carbohydrates
1. Monosaccharides (Simple Sugars)
These are the simplest carbohydrates – single sugar molecules that can’t be broken down further.
Examples:
- Glucose (C₆H₁₂O₆) – Blood sugar, immediate energy source
- Fructose – Found in fruits, sweetest natural sugar
- Galactose – Found in milk products
Memory Tip: Remember “GLU-FRU-GAL” – like three friends who are all simple and sweet!
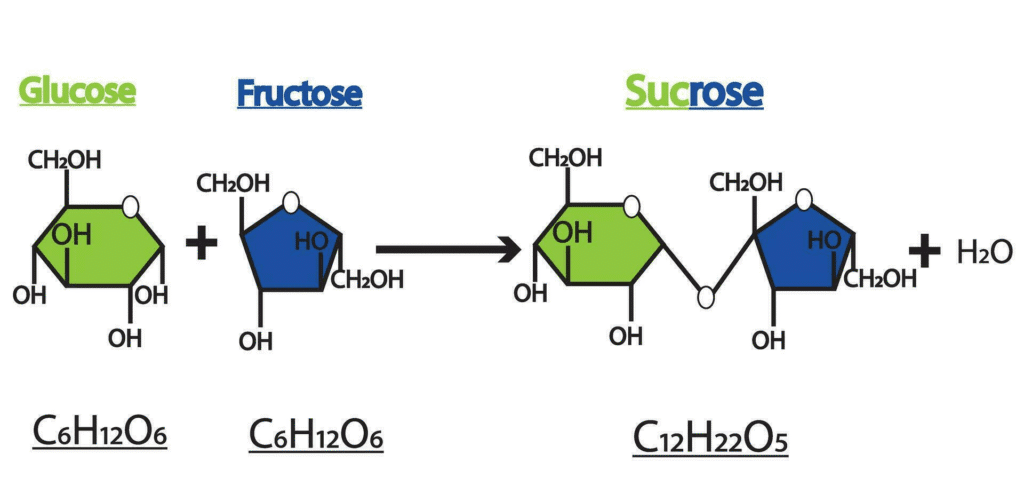
2. Disaccharides (Double Sugars)
Two monosaccharides joined together by a glycosidic bond.
Examples:
- Sucrose = Glucose + Fructose (table sugar)
- Lactose = Glucose + Galactose (milk sugar)
- Maltose = Glucose + Glucose (malt sugar)
Key Reaction:
Condensation: Monosaccharide + Monosaccharide → Disaccharide + Water
Hydrolysis: Disaccharide + Water → Monosaccharide + Monosaccharide
3. Polysaccharides (Complex Carbohydrates)
Long chains of monosaccharides linked together.
Examples:
- Starch – Plant energy storage (amylose and amylopectin)
- Glycogen – Animal energy storage (“animal starch”)
- Cellulose – Plant structural support (cell walls)
Functions of Carbohydrates
- Immediate Energy Source: Glucose provides quick energy for cellular respiration
- Energy Storage: Starch in plants, glycogen in animals
- Structural Support: Cellulose in plant cell walls
- Cell Recognition: Glycoproteins on cell surfaces
Testing for Carbohydrates
Benedict’s Test (Reducing Sugars):
- Add Benedict’s solution to sample
- Heat in water bath
- Positive result: Color change from blue → green → orange → brick red
Iodine Test (Starch):
- Add iodine solution to sample
- Positive result: Color change from orange → blue-black
Proteins: The Molecular Machines
What Are Proteins?
Proteins are complex molecules made up of amino acids. Think of amino acids as letters in an alphabet – just as 26 letters can create countless words, 20 different amino acids can create countless proteins, each with unique properties and functions.
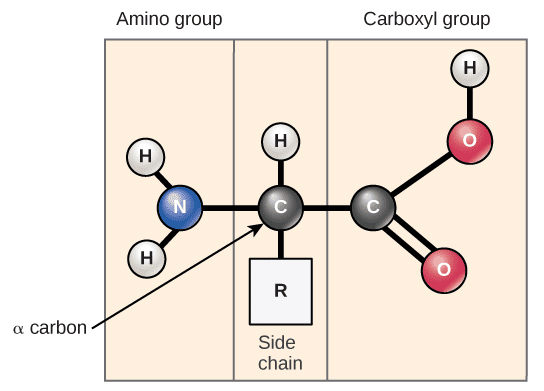
Basic Structure: All amino acids have:
- An amino group (-NH₂)
- A carboxyl group (-COOH)
- A variable R group (side chain)
- A central carbon atom
Protein Structure Levels
1. Primary Structure
The sequence of amino acids in the protein chain, held together by peptide bonds.
2. Secondary Structure
Local folding patterns like α-helices and β-pleated sheets, stabilized by hydrogen bonds.
3. Tertiary Structure
Overall 3D shape of the protein, maintained by various bonds and interactions.
4. Quaternary Structure
Multiple protein chains working together (like hemoglobin with its four subunits).
Key Protein Reactions
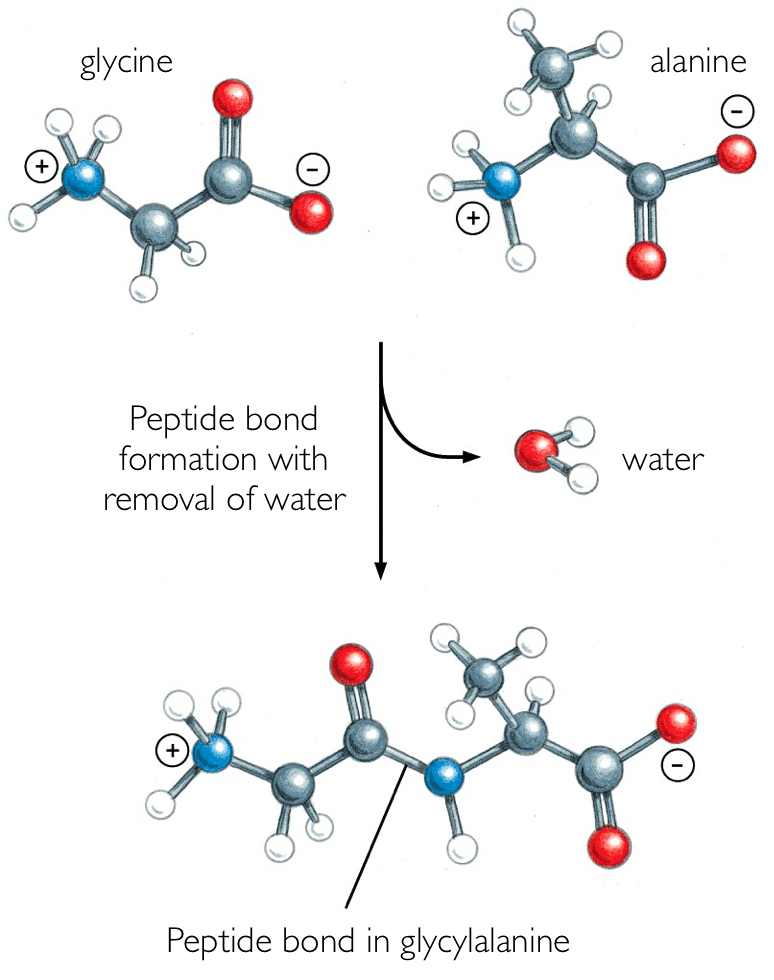
Peptide Bond Formation (Condensation):
Amino Acid + Amino Acid → Dipeptide + Water
Protein Hydrolysis:
Protein + Water → Amino Acids (catalyzed by enzymes)
Functions of Proteins
- Enzymes: Biological catalysts (like amylase, pepsin)
- Structural: Building materials (collagen, keratin)
- Transport: Carrying molecules (hemoglobin carries oxygen)
- Hormones: Chemical messengers (insulin)
- Antibodies: Immune system defense
- Storage: Storing amino acids (albumin in egg white)
Protein Testing
Biuret Test:
- Add Biuret solution to sample
- Positive result: Color change from blue → purple/lilac
Factors Affecting Protein Structure
Denaturation occurs when proteins lose their shape due to:
- High temperature
- Extreme pH changes
- Heavy metals
- Radiation
Real-life Example: When you cook an egg, heat denatures the proteins in egg white, changing it from clear and runny to white and solid!
Lipids: The Energy Warehouses
What Are Lipids?
Lipids are a diverse group of molecules that are mostly hydrophobic (water-repelling). Unlike other biological molecules, they don’t have a common structural unit, but they share the property of being largely composed of carbon and hydrogen atoms.
Types of Lipids
1. Fats and Oils (Triglycerides)
Made up of:
- One glycerol molecule
- Three fatty acid chains
Key Reaction:
Glycerol + 3 Fatty Acids ⇌ Triglyceride + 3 Water molecules
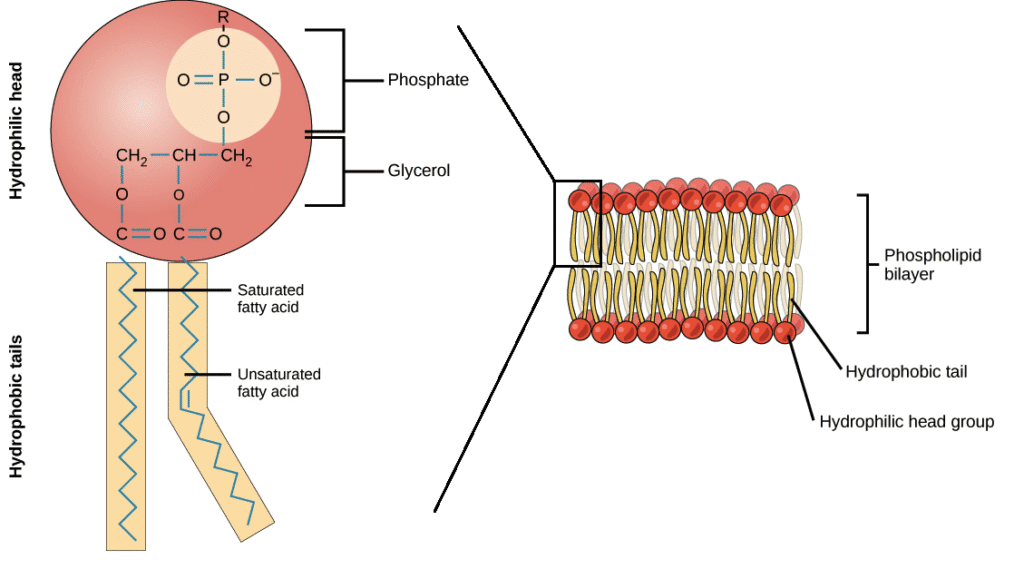
2. Phospholipids
Similar to triglycerides but with:
- One glycerol molecule
- Two fatty acid chains
- One phosphate group
This creates a molecule with a hydrophilic (water-loving) head and hydrophobic (water-hating) tails – perfect for cell membranes!
Saturated vs. Unsaturated Fats
Saturated Fats:
- No double bonds between carbon atoms
- Straight chains pack tightly together
- Solid at room temperature (butter, lard)
- Generally from animal sources
Unsaturated Fats:
- One or more double bonds create “kinks”
- Chains don’t pack tightly
- Liquid at room temperature (olive oil, fish oil)
- Generally from plant sources
Functions of Lipids
- Energy Storage: More than twice the energy per gram compared to carbohydrates
- Insulation: Subcutaneous fat keeps body warm
- Protection: Fat pads protect organs
- Cell Membranes: Phospholipids form membrane structure
- Signaling: Some hormones are lipid-based (testosterone, estrogen)
Testing for Lipids
Emulsion Test:
- Mix sample with ethanol, then add water
- Positive result: Milky white emulsion forms
Sudan III Test:
- Add Sudan III stain to sample
- Positive result: Red/orange coloration
Nucleic Acids: Life’s Instruction Manual
What Are Nucleic Acids?
Nucleic acids are the information storage molecules of life. They’re like libraries containing all the instructions needed to build and maintain an organism.
Types of Nucleic Acids
1. DNA (Deoxyribonucleic Acid)
- Double-stranded helix
- Contains genetic instructions
- Found mainly in the nucleus
- Uses bases: Adenine (A), Thymine (T), Guanine (G), Cytosine (C)
2. RNA (Ribonucleic Acid)
- Usually single-stranded
- Involved in protein synthesis
- Found in nucleus and cytoplasm
- Uses bases: Adenine (A), Uracil (U), Guanine (G), Cytosine (C)
Nucleotide Structure
Each nucleotide consists of:
- Phosphate group
- Pentose sugar (ribose in RNA, deoxyribose in DNA)
- Nitrogenous base
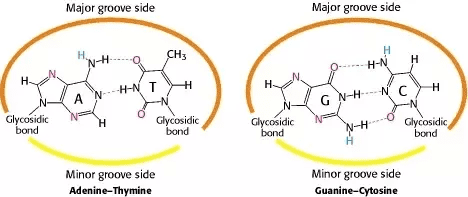
DNA Base Pairing Rules
- Adenine (A) pairs with Thymine (T) – 2 hydrogen bonds
- Guanine (G) pairs with Cytosine (C) – 3 hydrogen bonds
Memory Tip: “Apples in the Tree” (A-T) and “Cars in the Garage” (C-G)
Functions of Nucleic Acids
- Information Storage: DNA stores genetic code
- Information Transfer: RNA carries genetic information
- Protein Synthesis: RNA helps build proteins
- Heredity: DNA passes traits to offspring
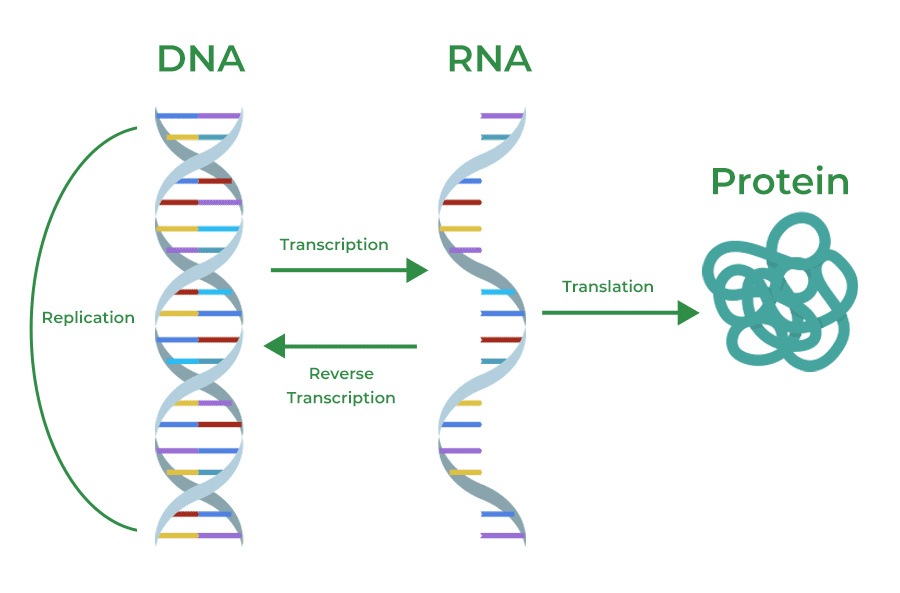
How Biological Molecules Work Together
Biological molecules don’t work in isolation – they’re part of an interconnected system. Here’s how they collaborate:
Energy Production: Carbohydrates provide immediate energy, while lipids store long-term energy. Proteins (enzymes) catalyze the reactions that release this energy.
Cell Structure: Proteins provide structural framework, lipids form membranes, and polysaccharides like cellulose provide plant support.
Information Flow: DNA stores information, RNA transfers it, and proteins act on it to create cellular structures and carry out functions.
Common Exam Questions and How to Tackle Them
Question Type 1: Structure and Function
“Explain how the structure of starch makes it suitable for energy storage.”
Approach:
- Identify key structural features
- Link each feature to its functional advantage
- Use specific scientific terminology
Model Answer: Starch is a polysaccharide composed of glucose monomers joined by glycosidic bonds. Its branched structure (amylopectin) allows for rapid hydrolysis when energy is needed. Being insoluble, it doesn’t affect water potential in cells. Its large size prevents it from leaving the cell, making it ideal for storage.
Question Type 2: Testing and Identification
“Describe how you would test a food sample for the presence of protein.”
Approach:
- State the test name
- Describe the procedure step-by-step
- Give the positive result
- Explain what the result indicates
Question Type 3: Comparison Questions
“Compare the structure and function of DNA and RNA.”
Approach:
- Create a systematic comparison
- Cover structure first, then function
- Use a table format mentally
- Include specific examples
Quick Revision Notes
Carbohydrates Summary
- Elements: C, H, O (1:2:1 ratio)
- Monomers: Monosaccharides (glucose, fructose, galactose)
- Polymers: Polysaccharides (starch, glycogen, cellulose)
- Functions: Energy, storage, structure
- Tests: Benedict’s (reducing sugars), Iodine (starch)
Proteins Summary
- Elements: C, H, O, N, S
- Monomers: Amino acids (20 types)
- Bonds: Peptide bonds
- Functions: Enzymes, structure, transport, hormones
- Test: Biuret test (blue → purple)
Lipids Summary
- Elements: C, H, O (more C and H than carbohydrates)
- Types: Triglycerides, phospholipids
- Properties: Hydrophobic, energy-rich
- Functions: Energy storage, insulation, membranes
- Tests: Emulsion test, Sudan III
Nucleic Acids Summary
- Elements: C, H, O, N, P
- Types: DNA (double helix), RNA (single strand)
- Monomers: Nucleotides
- Functions: Information storage and transfer
- Base Pairing: A-T/U, G-C
Key Formulas and Equations Box
Important Chemical Formulas:
- Glucose: C₆H₁₂O₆
- General carbohydrate: (CH₂O)ₙ
- Sucrose: C₁₂H₂₂O₁₁
Key Reactions:
- Condensation: Monomer + Monomer → Polymer + Water
- Hydrolysis: Polymer + Water → Monomer + Monomer
- Peptide bond formation: R-NH₂ + HOOC-R → R-NH-CO-R + H₂O
Common Mistakes to Avoid
- Confusing condensation and hydrolysis – Remember: condensation removes water, hydrolysis adds water
- Mixing up RNA and DNA bases – DNA has Thymine, RNA has Uracil
- Forgetting test colors – Practice until Benedict’s blue→red and Biuret blue→purple are automatic
- Not linking structure to function – Always explain WHY a structure makes the molecule suitable for its role
- Ignoring the role of water – Water is crucial in both building (hydrolysis) and breaking down (condensation) biological molecules
Test Yourself: Practice Questions
- Explain why glucose is described as a reducing sugar but sucrose is not.
- Compare the energy storage molecules glycogen and starch in terms of structure and location.
- Describe how the structure of phospholipids makes them suitable for forming cell membranes.
- Explain the importance of the base pairing rules in DNA replication.
- Outline the different levels of protein structure and the bonds involved in each.
Study Tips for Success
Memory Techniques
- Acronyms: Create memorable phrases like “Good Friends Go Cycling” for Glucose, Fructose, Galactose, Cellulose
- Visual associations: Draw simple diagrams repeatedly until they become automatic
- Story method: Create narratives about molecules going on adventures
Exam Preparation Strategy
- Master the basics first – Ensure you know all monomers and polymers
- Practice drawing structures – Especially glucose, amino acids, and nucleotides
- Learn test procedures thoroughly – Examiners love testing practical knowledge
- Connect topics – Link biological molecules to other topics like respiration and photosynthesis
Time Management
- Spend extra time on proteins – they’re the most complex topic
- Practice timed questions regularly
- Focus on your weak areas but don’t neglect strengths
Looking Ahead: Connecting to Other Topics
Understanding biological molecules is crucial for success in other IGCSE Biology topics:
- Topic 5 (Enzymes): All enzymes are proteins – your protein knowledge will be essential
- Topic 12 (Respiration): Glucose breakdown and ATP synthesis build on your carbohydrate understanding
- Topic 13 (Photosynthesis): Glucose production and starch storage connect directly to this topic
- Topic 17 (Inheritance): DNA structure knowledge is fundamental for genetics
Conclusion: You’ve Got This!
Congratulations! You’ve just completed a comprehensive journey through the fascinating world of biological molecules. From the simple sugars that fuel your brain while studying, to the complex proteins that make your muscles contract, to the DNA that makes you uniquely you – these molecules are the foundation of all life.
Remember, biology isn’t just about memorizing facts and formulas. It’s about understanding the incredible complexity and elegance of living systems. Every time you eat a meal, your body breaks down carbohydrates, proteins, and lipids into their component parts and rebuilds them into the molecules you need. Every time you grow, repair an injury, or even just think a thought, these biological molecules are working together in perfect harmony.
As you prepare for your IGCSE Biology exam, keep this big picture in mind. Yes, you need to know that Benedict’s solution turns from blue to brick-red, and yes, you need to remember that DNA uses thymine while RNA uses uracil. But more importantly, understand that you’re learning about the very essence of what makes life possible.
Your Next Steps:
- Review this guide section by section over the coming weeks
- Practice drawing molecular structures until they become second nature
- Work through past paper questions, focusing on explaining structure-function relationships
- Connect these concepts to other biology topics as you study them
- Don’t hesitate to ask for help when concepts seem challenging
You have all the tools you need to excel in this topic. The molecules that make up your brain are the same ones you’re studying – how amazing is that? Trust in your ability to understand these concepts, stay curious about the world around you, and remember that every expert was once a beginner.
Recommended –

1 thought on “IGCSE (Cambridge) Biology Topic 4: Biological Molecules | Your Complete Study Guide”