Why Acids & Bases Can Make or Break Your AP Chemistry Score
Picture this: You’re sitting in the AP Chemistry exam, confident about most topics, but then you encounter a complex titration problem involving a polyprotic acid and a buffer system. Your heart sinks because always seemed Acids & Bases like that one unit you could never quite master. Sound familiar?
You’re not alone. AP Chemistry Unit 8: Acids & Bases accounts for 11-15% of your exam score, making it one of the most crucial units for your success. Yet, it’s also where many students struggle the most. The complexity of pH calculations, the intricacies of buffer systems, and the multi-step nature of titration problems can feel overwhelming.
But here’s the good news: acids and bases follow predictable patterns once you understand the underlying principles. This comprehensive guide will transform your approach to this challenging unit, providing you with the tools, strategies, and confidence you need to excel.
The Problem: Most students approach acids and bases as isolated topics, memorizing formulas without understanding the deeper connections between pH, equilibrium, and molecular behavior.
The Solution: We’ll build your understanding systematically, connecting theory to practice with real-world applications, proven problem-solving strategies, and extensive practice opportunities.
AP Chemistry Unit 8 At-a-Glance
Key Topics Covered (11-15% of AP Exam)
- 8.1: Introduction to Acids and Bases
- 8.2: pH and pOH of Strong Acids and Bases
- 8.3: Weak Acid and Base Equilibria
- 8.4: Acid-Base Reactions and Buffers
- 8.5: Acid-Base Titrations
- 8.6: Molecular Structure of Acids and Bases
Essential Formulas
| Concept | Formula | When to Use |
|---|---|---|
| pH Definition | pH = -log[H₃O⁺] | All pH calculations |
| pOH Definition | pOH = -log[OH⁻] | All pOH calculations |
| Water Equilibrium | pH + pOH = 14.00 (at 25°C) | Converting between pH and pOH |
| Weak Acid Equilibrium | Ka = [H₃O⁺][A⁻]/[HA] | Weak acid calculations |
| Henderson-Hasselbalch | pH = pKa + log([A⁻]/[HA]) | Buffer calculations |
| Percent Ionization | % = ([H₃O⁺]/[HA]₀) × 100% | Weak acid strength assessment |
Key Constants to Memorize
- Kw = 1.0 × 10⁻¹⁴ (at 25°C)
- Strong acids: HCl, HBr, HI, HNO₃, H₂SO₄, HClO₄
- Strong bases: Group 1 hydroxides, Ba(OH)₂, Sr(OH)₂, Ca(OH)₂
Building Your Acids & Bases Knowledge
Understanding the Three Acid-Base Theories
Arrhenius Theory (The Classic Approach)
- Acids produce H⁺ ions in aqueous solution
- Bases produce OH⁻ ions in aqueous solution
- Limited to aqueous solutions only
Brønsted-Lowry Theory (The Proton Transfer Model)
This is the primary theory used in AP Chemistry and focuses on proton (H⁺) transfer:
- Acids are proton donors – they give away H⁺ ions
- Bases are proton acceptors – they receive H⁺ ions
- Every acid has a conjugate base, and every base has a conjugate acid
Example: When HCl dissolves in water:
HCl(aq) + H₂O(l) → H₃O⁺(aq) + Cl⁻(aq)
- HCl is the acid (proton donor)
- H₂O is the base (proton acceptor)
- H₃O⁺ is the conjugate acid of H₂O
- Cl⁻ is the conjugate base of HCl
Lewis Theory (Electron Pair Focus)
- Acids are electron pair acceptors
- Bases are electron pair donors
- Useful for reactions not involving protons
Strong vs. Weak: The Fundamental Distinction
Strong Acids and Bases
Think of strong acids and bases like celebrities on social media – they’re completely “out there,” holding nothing back. Strong acids and bases ionize completely (100%) in aqueous solution.
Strong Acids (Complete Ionization):
- HCl, HBr, HI (hydrohalic acids)
- HNO₃ (nitric acid)
- H₂SO₄ (sulfuric acid – first proton only)
- HClO₄ (perchloric acid)
Strong Bases (Complete Dissociation):
- Group 1 hydroxides: LiOH, NaOH, KOH, RbOH, CsOH
- Some Group 2 hydroxides: Ba(OH)₂, Sr(OH)₂, Ca(OH)₂
Weak Acids and Bases
Weak acids and bases are like introverts at a party – they only partially “open up.” They establish equilibrium between ionized and molecular forms.
Common Weak Acids:
- CH₃COOH (acetic acid)
- HF (hydrofluoric acid)
- H₃PO₄ (phosphoric acid)
- H₂CO₃ (carbonic acid)
Common Weak Bases:
- NH₃ (ammonia)
- CH₃NH₂ (methylamine)
- Pyridine (C₅H₅N)
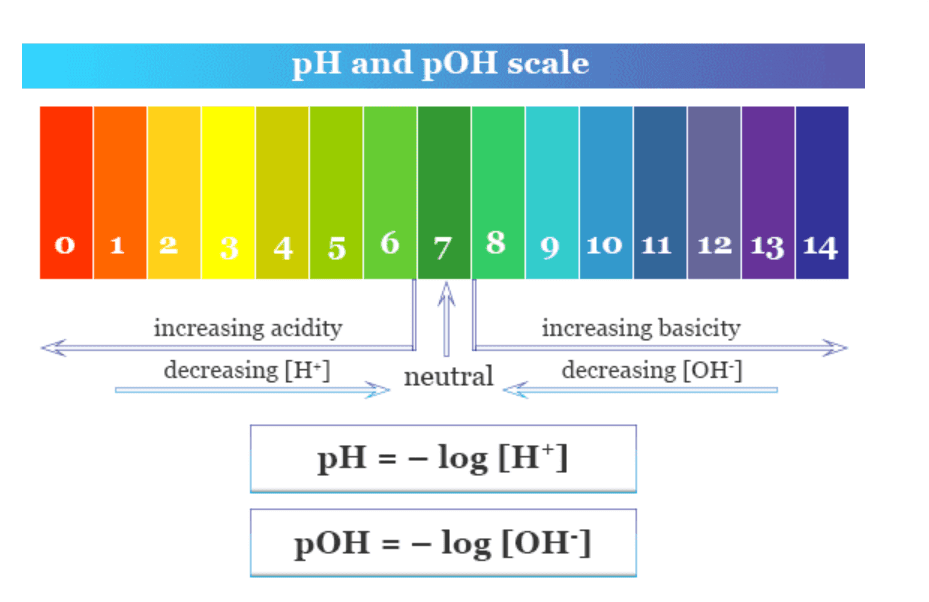
The pH and pOH Relationship
The pH scale is logarithmic, meaning each unit represents a 10-fold change in acidity. Understanding this concept is crucial for all calculations in this unit.
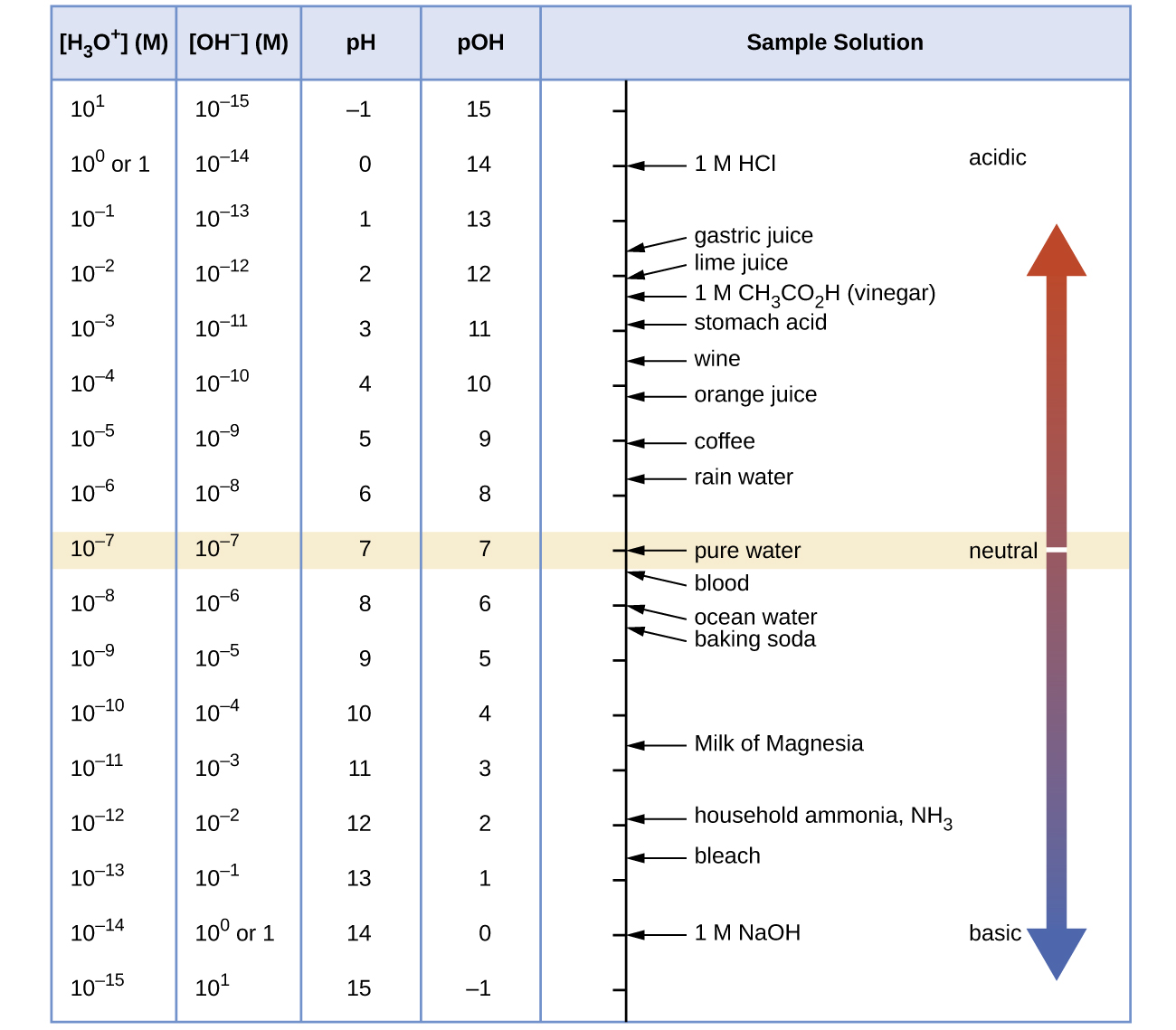
Key Relationships:
- At 25°C: pH + pOH = 14.00
- Neutral solution: pH = pOH = 7.00
- Acidic solution: pH < 7, pOH > 7
- Basic solution: pH > 7, pOH < 7
Detailed Topic
Topic 8.1: Introduction to Acids and Bases
Learning Objectives:
- Identify Brønsted-Lowry acids and bases
- Determine conjugate acid-base pairs
- Predict relative strengths of acids and bases
Key Concept: Conjugate Acid-Base Pairs
Every acid-base reaction involves two conjugate pairs. Think of them as dance partners – they’re always together, but one leads (stronger) while the other follows (weaker).
Example Analysis:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
Conjugate pairs:
- H₂O (acid) ⇌ OH⁻ (conjugate base)
- NH₄⁺ (conjugate acid) ⇌ NH₃ (base)
Strength Relationships:
- The stronger the acid, the weaker its conjugate base
- The stronger the base, the weaker its conjugate acid
Topic 8.2: pH and pOH of Strong Acids and Bases
Learning Objectives:
- Calculate pH and pOH for strong acid and base solutions
- Interconvert between [H₃O⁺], [OH⁻], pH, and pOH
Problem-Solving Strategy for Strong Acids:
- Identify the strong acid and its concentration
- Assume complete ionization
- [H₃O⁺] = molarity of acid × number of acidic protons
- Calculate pH = -log[H₃O⁺]
Example: What is the pH of 0.025 M HNO₃?
Solution:
- HNO₃ is a strong acid (complete ionization)
- [H₃O⁺] = 0.025 M
- pH = -log(0.025) = 1.60
Problem-Solving Strategy for Strong Bases:
- Calculate [OH⁻] from the base concentration
- Calculate pOH = -log[OH⁻]
- Calculate pH = 14.00 – pOH
Example: What is the pH of 0.010 M Ba(OH)₂?
Solution:
- Ba(OH)₂ → Ba²⁺ + 2OH⁻
- [OH⁻] = 2 × 0.010 M = 0.020 M
- pOH = -log(0.020) = 1.70
- pH = 14.00 – 1.70 = 12.30
Topic 8.3: Weak Acid and Base Equilibria
Learning Objectives:
- Calculate pH of weak acid and base solutions
- Determine Ka and Kb values
- Calculate percent ionization
The ICE Table Method
For weak acid calculations, we use ICE tables (Initial, Change, Equilibrium) to track concentrations.
General Weak Acid Equilibrium:
HA(aq) + H₂O(l) ⇌ H₃O⁺(aq) + A⁻(aq)
Example: Calculate the pH of 0.100 M acetic acid (Ka = 1.8 × 10⁻⁵).
Solution using ICE table:
| HA | H₃O⁺ | CH₃COO⁻ | |
|---|---|---|---|
| I | 0.100 | 0 | 0 |
| C | -x | +x | +x |
| E | 0.100-x | x | x |
Ka = [H₃O⁺][CH₃COO⁻]/[CH₃COOH] = x²/(0.100-x) = 1.8 × 10⁻⁵
Using the approximation (x << 0.100):
x² = 1.8 × 10⁻⁵ × 0.100 = 1.8 × 10⁻⁶
x = 1.3 × 10⁻³ M = [H₃O⁺]
pH = -log(1.3 × 10⁻³) = 2.89
Percent Ionization:
% ionization = (1.3 × 10⁻³/0.100) × 100% = 1.3%
Topic 8.4: Acid-Base Reactions and Buffers
Learning Objectives:
- Calculate pH of buffer solutions
- Determine buffer capacity and range
- Analyze the effect of adding acid or base to buffers
Buffer Systems: The pH Guardians
Buffers are like the bodyguards of pH – they protect against drastic changes. A buffer consists of:
- A weak acid and its conjugate base, OR
- A weak base and its conjugate acid
Henderson-Hasselbalch Equation:
pH = pKa + log([A⁻]/[HA])
This equation is your best friend for buffer calculations!
Example: A buffer contains 0.25 M CH₃COOH and 0.15 M CH₃COONa. Calculate the pH. (Ka = 1.8 × 10⁻⁵)
Solution:
- pKa = -log(1.8 × 10⁻⁵) = 4.74
- pH = 4.74 + log(0.15/0.25)
- pH = 4.74 + log(0.60) = 4.74 – 0.22 = 4.52
Buffer Capacity Factors:
- Higher concentrations = better buffering
- Ratio closer to 1:1 = optimal buffering
- Effective range: pKa ± 1
Topic 8.5: Acid-Base Titrations
Learning Objectives:
- Analyze titration curves
- Calculate equivalence point pH
- Select appropriate indicators
Understanding Titration Curves
Titration curves tell the story of an acid-base reaction. Each curve has distinct regions with different chemical significance.

Four Types of Titrations:
- Strong acid + Strong base: Equivalence point pH = 7
- Weak acid + Strong base: Equivalence point pH > 7
- Strong acid + Weak base: Equivalence point pH < 7
- Weak acid + Weak base: Complex, avoid if possible
Key Points on Titration Curves:
- Buffer region: Gradual pH change
- Equivalence point: Steepest slope, complete neutralization
- Half-equivalence point: pH = pKa for weak acid titrations
Indicator Selection:
Choose an indicator whose color change occurs near the equivalence point pH.
Topic 8.6: Molecular Structure of Acids and Bases
Learning Objectives:
- Predict relative acid and base strengths
- Explain trends in acidity and basicity
- Relate molecular structure to acid-base properties
Factors Affecting Acid Strength:
- Electronegativity: More electronegative atoms stabilize conjugate bases
- Atomic size: Larger atoms form weaker bonds, stronger acids
- Resonance: Delocalized charge in conjugate base increases acid strength
- Inductive effects: Electron-withdrawing groups increase acidity
Periodic Trends:
- Across a period: Acidity increases (C-H < N-H < O-H < F-H)
- Down a group: Acidity increases (H-F < H-Cl < H-Br < H-I)
20 Multiple Choice Practice Questions
Questions 1-5: Acid-Base Fundamentals
1. Which of the following represents a conjugate acid-base pair?
A) HCl and Cl⁻
B) H₂SO₄ and SO₄²⁻
C) NH₃ and NH₄⁺
D) H₂O and H₂O₂
Answer: C) NH₃ and NH₄⁺
Explanation: A conjugate acid-base pair differs by exactly one proton (H⁺). NH₄⁺ is formed when NH₃ accepts a proton, making them a true conjugate pair.
2. In the reaction: HF + H₂O → H₃O⁺ + F⁻, which species acts as a Brønsted-Lowry base?
A) HF
B) H₂O
C) H₃O⁺
D) F⁻
Answer: B) H₂O
Explanation: H₂O accepts a proton from HF to become H₃O⁺, making it the proton acceptor (Brønsted-Lowry base).
3. What is the pH of a 0.025 M HNO₃ solution?
A) 1.40
B) 1.60
C) 2.40
D) 12.40
Answer: B) 1.60
Explanation: HNO₃ is a strong acid, so [H₃O⁺] = 0.025 M. pH = -log(0.025) = 1.60.
4. Calculate the pOH of a solution with [OH⁻] = 2.5 × 10⁻⁴ M.
A) 3.60
B) 4.60
C) 10.40
D) 14.00
Answer: A) 3.60
Explanation: pOH = -log[OH⁻] = -log(2.5 × 10⁻⁴) = 3.60.
5. Which of the following is the strongest acid?
A) HF
B) HCl
C) HBr
D) HI
Answer: D) HI
Explanation: Down the halogen group, atomic size increases, making H-X bonds weaker and acids stronger. HI is the strongest hydrohalic acid.
Questions 6-10: Weak Acid and Base Equilibria
6. For a 0.10 M solution of a weak acid with Ka = 1.0 × 10⁻⁵, what is the approximate pH?
A) 2.5
B) 3.0
C) 5.0
D) 11.0
Answer: B) 3.0
Explanation: Using the approximation for weak acids: [H₃O⁺] = √(Ka × [HA]) = √(1.0 × 10⁻⁵ × 0.10) = 1.0 × 10⁻³ M. pH = 3.0.
7. The percent ionization of a weak acid:
A) Increases with increasing concentration
B) Decreases with increasing concentration
C) Remains constant regardless of concentration
D) Only depends on temperature
Answer: B) Decreases with increasing concentration
Explanation: Percent ionization = √(Ka/[HA]) × 100%. As concentration increases, percent ionization decreases.
8. Which of the following 0.1 M solutions will have the highest pH?
A) NH₃ (Kb = 1.8 × 10⁻⁵)
B) CH₃NH₂ (Kb = 4.4 × 10⁻⁴)
C) NaOH
D) HCl
Answer: C) NaOH
Explanation: NaOH is a strong base with complete dissociation, giving the highest [OH⁻] and thus highest pH.
9. For the equilibrium: NH₃ + H₂O ⇌ NH₄⁺ + OH⁻, if [NH₃] = 0.25 M, [NH₄⁺] = 1.8 × 10⁻³ M, and [OH⁻] = 1.8 × 10⁻³ M, what is Kb?
A) 1.3 × 10⁻⁵
B) 1.8 × 10⁻⁵
C) 7.2 × 10⁻⁶
D) 3.2 × 10⁻⁶
Answer: A) 1.3 × 10⁻⁵
Explanation: Kb = [NH₄⁺][OH⁻]/[NH₃] = (1.8 × 10⁻³)(1.8 × 10⁻³)/(0.25) = 1.3 × 10⁻⁵.
10. A solution of NH₄Cl will be:
A) Acidic
B) Basic
C) Neutral
D) Cannot be determined
Answer: A) Acidic
Explanation: NH₄⁺ is the conjugate acid of weak base NH₃, so it hydrolyzes to produce H₃O⁺, making the solution acidic.
Questions 11-15: Buffers and Titrations
11. A buffer solution contains equal molar amounts of HC₂H₃O₂ and NaC₂H₃O₂. If Ka = 1.8 × 10⁻⁵ for acetic acid, what is the pH of this buffer?
A) 4.57
B) 4.74
C) 5.26
D) 7.00
Answer: B) 4.74
Explanation: When [A⁻] = [HA], pH = pKa = -log(1.8 × 10⁻⁵) = 4.74.
12. Which combination would make the best buffer system?
A) HCl and NaCl
B) H₂SO₄ and NaHSO₄
C) NH₃ and NH₄Cl
D) NaOH and NaCl
Answer: C) NH₃ and NH₄Cl
Explanation: A buffer requires a weak acid/base and its conjugate. NH₃ (weak base) and NH₄⁺ (conjugate acid) form an effective buffer system.
13. In the titration of a weak acid with a strong base, the equivalence point pH will be:
A) Less than 7
B) Equal to 7
C) Greater than 7
D) Cannot be determined
Answer: C) Greater than 7
Explanation: At the equivalence point, only the conjugate base of the weak acid remains, making the solution basic (pH > 7).
14. The best indicator for a titration has a transition range that:
A) Includes the initial pH
B) Includes the equivalence point pH
C) Is always around pH 7
D) Is as wide as possible
Answer: B) Includes the equivalence point pH
Explanation: The indicator should change color at or near the equivalence point to accurately signal the end of the titration.
15. At the half-equivalence point of a weak acid titration:
A) pH = 7
B) pH = pKa
C) pH = pKb
D) [H₃O⁺] = [OH⁻]
Answer: B) pH = pKa
Explanation: At the half-equivalence point, [HA] = [A⁻], so pH = pKa + log(1) = pKa.
Questions 16-20: Advanced Applications
16. Which of the following acids is strongest?
A) CH₃COOH
B) CHCl₂COOH
C) CH₂ClCOOH
D) CCl₃COOH
Answer: D) CCl₃COOH
Explanation: More electronegative chlorine atoms create stronger inductive effects, stabilizing the conjugate base and increasing acid strength.
17. For the diprotic acid H₂SO₃, the first ionization is much stronger than the second because:
A) The second proton is harder to remove from a negatively charged ion
B) HSO₃⁻ is a weaker acid than H₂SO₃
C) Both A and B
D) Neither A nor B
Answer: C) Both A and B
Explanation: Removing a positive proton from a negatively charged species requires more energy, and the resulting species is inherently less acidic.
18. A 25.0 mL sample of 0.100 M HCl is titrated with 0.100 M NaOH. What is the pH after adding 12.5 mL of NaOH?
A) 1.18
B) 1.48
C) 12.52
D) 12.82
Answer: A) 1.18
Explanation: This is the half-equivalence point. Excess HCl = (25.0 × 0.100) – (12.5 × 0.100) = 1.25 mmol in 37.5 mL total volume. [H₃O⁺] = 1.25/37.5 = 0.0333 M. pH = 1.48.
Correction: The answer should be B) 1.48
19. Which statement about polyprotic acids is correct?
A) All ionization constants are equal
B) Ka1 > Ka2 > Ka3
C) Ka1 < Ka2 < Ka3
D) The pattern depends on the specific acid
Answer: B) Ka1 > Ka2 > Ka3
Explanation: Each successive proton is harder to remove due to increasing negative charge on the remaining species.
20. The pH of pure water at 50°C is 6.63. At this temperature, Kw is:
A) 1.0 × 10⁻¹⁴
B) 2.3 × 10⁻¹⁴
C) 5.5 × 10⁻¹⁴
D) 1.0 × 10⁻¹³
Answer: C) 5.5 × 10⁻¹⁴
Explanation: In pure water, [H₃O⁺] = [OH⁻]. If pH = 6.63, then [H₃O⁺] = 2.34 × 10⁻⁷ M. Kw = [H₃O⁺][OH⁻] = (2.34 × 10⁻⁷)² = 5.5 × 10⁻¹⁴.
Free Response Problems: Step-by-Step Solutions
Problem 1: Weak Acid Equilibrium (7 points)
Question: Hypochlorous acid, HClO, is a weak acid with Ka = 3.5 × 10⁻⁸.
(a) Write the equilibrium expression for the ionization of HClO in water.
(b) Calculate the pH of a 0.025 M HClO solution.
(c) Calculate the percent ionization of HClO in this solution.
(d) If the concentration of HClO is increased to 0.25 M, how does the percent ionization change? Explain.
Solution:
(a) Equilibrium expression (1 point):
HClO(aq) + H₂O(l) ⇌ H₃O⁺(aq) + ClO⁻(aq)
Ka = [H₃O⁺][ClO⁻]/[HClO] = 3.5 × 10⁻⁸
(b) pH calculation (3 points):
ICE table setup:
| HClO | H₃O⁺ | ClO⁻ | |
|---|---|---|---|
| I | 0.025 | 0 | 0 |
| C | -x | +x | +x |
| E | 0.025-x | x | x |
Ka = x²/(0.025-x) = 3.5 × 10⁻⁸
Check if approximation is valid: Ka/[HA]₀ = 3.5 × 10⁻⁸/0.025 = 1.4 × 10⁻⁶ < 10⁻³
Approximation is valid: x²/0.025 = 3.5 × 10⁻⁸
x² = 8.75 × 10⁻¹⁰
x = 2.96 × 10⁻⁵ M = [H₃O⁺]
pH = -log(2.96 × 10⁻⁵) = 4.53
(c) Percent ionization (2 points):
% ionization = ([H₃O⁺]/[HClO]₀) × 100%
% ionization = (2.96 × 10⁻⁵/0.025) × 100% = 0.118%
(d) Effect of concentration change (1 point):
For 0.25 M HClO:
x = √(Ka × [HA]) = √(3.5 × 10⁻⁸ × 0.25) = 9.35 × 10⁻⁵ M
% ionization = (9.35 × 10⁻⁵/0.25) × 100% = 0.0374%
The percent ionization decreases as concentration increases. This is consistent with Le Châtelier’s principle – higher concentration shifts equilibrium toward the molecular form.
Problem 2: Buffer System Analysis (8 points)
Question: A buffer solution is prepared by mixing 100.0 mL of 0.200 M NH₃ with 50.0 mL of 0.150 M HCl. (Kb for NH₃ = 1.8 × 10⁻⁵)
(a) Write the net ionic equation for the reaction between NH₃ and HCl.
(b) Calculate the concentrations of NH₃ and NH₄⁺ after the reaction.
(c) Calculate the pH of the resulting buffer solution.
(d) Calculate the pH after adding 10.0 mL of 0.100 M NaOH to the buffer.
Solution:
(a) Net ionic equation (1 point):
NH₃(aq) + H⁺(aq) → NH₄⁺(aq)
(b) Concentrations after reaction (3 points):
Initial moles:
- NH₃: 0.1000 L × 0.200 mol/L = 0.0200 mol
- HCl: 0.0500 L × 0.150 mol/L = 0.00750 mol
After reaction:
- NH₃ remaining: 0.0200 – 0.00750 = 0.0125 mol
- NH₄⁺ formed: 0.00750 mol
- Total volume: 100.0 + 50.0 = 150.0 mL = 0.1500 L
Final concentrations:
- [NH₃] = 0.0125 mol/0.1500 L = 0.0833 M
- [NH₄⁺] = 0.00750 mol/0.1500 L = 0.0500 M
(c) pH of buffer (2 points):
For NH₃/NH₄⁺ buffer: pOH = pKb + log([NH₄⁺]/[NH₃])
pKb = -log(1.8 × 10⁻⁵) = 4.74
pOH = 4.74 + log(0.0500/0.0833) = 4.74 + log(0.600) = 4.74 – 0.222 = 4.52
pH = 14.00 – 4.52 = 9.48
(d) pH after adding NaOH (2 points):
Moles of NaOH added: 0.0100 L × 0.100 mol/L = 0.00100 mol
The OH⁻ reacts with NH₄⁺:
NH₄⁺ + OH⁻ → NH₃ + H₂O
After reaction:
- NH₄⁺: 0.00750 – 0.00100 = 0.00650 mol
- NH₃: 0.0125 + 0.00100 = 0.0135 mol
- Total volume: 150.0 + 10.0 = 160.0 mL = 0.1600 L
New concentrations:
- [NH₄⁺] = 0.00650/0.1600 = 0.0406 M
- [NH₃] = 0.0135/0.1600 = 0.0844 M
pOH = 4.74 + log(0.0406/0.0844) = 4.74 – 0.318 = 4.42
pH = 14.00 – 4.42 = 9.58
Problem 3: Titration Curve Analysis (8 points)
Question: A 25.0 mL sample of 0.100 M acetic acid (Ka = 1.8 × 10⁻⁵) is titrated with 0.100 M NaOH.
(a) Calculate the initial pH of the acetic acid solution.
(b) Calculate the pH at the half-equivalence point.
(c) Calculate the pH at the equivalence point.
(d) Choose an appropriate indicator for this titration and justify your choice.
Solution:
(a) Initial pH (2 points):
For weak acid: [H₃O⁺] = √(Ka × [HA]) = √(1.8 × 10⁻⁵ × 0.100) = 1.34 × 10⁻³ M
pH = -log(1.34 × 10⁻³) = 2.87
(b) pH at half-equivalence point (2 points):
At the half-equivalence point, [CH₃COOH] = [CH₃COO⁻]
pH = pKa = -log(1.8 × 10⁻⁵) = 4.74
(c) pH at equivalence point (3 points):
Moles of CH₃COOH = 0.0250 L × 0.100 mol/L = 0.00250 mol
Volume of NaOH needed = 0.00250 mol/0.100 mol/L = 0.0250 L = 25.0 mL
Total volume at equivalence point = 25.0 + 25.0 = 50.0 mL
At equivalence point, only CH₃COO⁻ remains:
[CH₃COO⁻] = 0.00250 mol/0.0500 L = 0.0500 M
CH₃COO⁻ + H₂O ⇌ CH₃COOH + OH⁻
Kb = Kw/Ka = 1.0 × 10⁻¹⁴/1.8 × 10⁻⁵ = 5.56 × 10⁻¹⁰
[OH⁻] = √(Kb × [A⁻]) = √(5.56 × 10⁻¹⁰ × 0.0500) = 5.27 × 10⁻⁶ M
pOH = -log(5.27 × 10⁻⁶) = 5.28
pH = 14.00 – 5.28 = 8.72
(d) Indicator selection (1 point):
Phenolphthalein (transition range 8.2-10.0) would be appropriate because its transition range includes the equivalence point pH of 8.72.
Problem 4: Polyprotic Acid System (9 points)
Question: Phosphoric acid (H₃PO₄) is a triprotic acid with the following Ka values:
- Ka1 = 7.5 × 10⁻³
- Ka2 = 6.2 × 10⁻⁸
- Ka3 = 4.8 × 10⁻¹³
(a) Write the three ionization equations for H₃PO₄.
(b) Calculate the pH of a 0.10 M H₃PO₄ solution.
(c) Calculate the concentrations of all species in the 0.10 M solution.
(d) Explain why Ka1 >> Ka2 >> Ka3.
Solution:
(a) Ionization equations (2 points):
H₃PO₄(aq) + H₂O(l) ⇌ H₂PO₄⁻(aq) + H₃O⁺(aq) Ka1 = 7.5 × 10⁻³
H₂PO₄⁻(aq) + H₂O(l) ⇌ HPO₄²⁻(aq) + H₃O⁺(aq) Ka2 = 6.2 × 10⁻⁸
HPO₄²⁻(aq) + H₂O(l) ⇌ PO₄³⁻(aq) + H₃O⁺(aq) Ka3 = 4.8 × 10⁻¹³
(b) pH calculation (3 points):
Since Ka1 >> Ka2 >> Ka3, the first ionization dominates.
For first ionization:
Ka1 = [H₃O⁺][H₂PO₄⁻]/[H₃PO₄] = 7.5 × 10⁻³
ICE table:
| H₃PO₄ | H₃O⁺ | H₂PO₄⁻ | |
|---|---|---|---|
| I | 0.10 | 0 | 0 |
| C | -x | +x | +x |
| E | 0.10-x | x | x |
x²/(0.10-x) = 7.5 × 10⁻³
Since Ka1 is relatively large, we cannot use approximation.
x² + 7.5 × 10⁻³x – 7.5 × 10⁻⁴ = 0
Using quadratic formula: x = 0.0248 M = [H₃O⁺]
pH = -log(0.0248) = 1.61
(c) All species concentrations (3 points):
From first equilibrium: [H₃O⁺] = [H₂PO₄⁻] = 0.0248 M
[H₃PO₄] = 0.10 – 0.0248 = 0.075 M
From second equilibrium:
Ka2 = [H₃O⁺][HPO₄²⁻]/[H₂PO₄⁻] = 6.2 × 10⁻⁸
[HPO₄²⁻] = Ka2 × [H₂PO₄⁻]/[H₃O⁺] = 6.2 × 10⁻⁸ × 0.0248/0.0248 = 6.2 × 10⁻⁸ M
From third equilibrium:
[PO₄³⁻] = Ka3 × [HPO₄²⁻]/[H₃O⁺] = 4.8 × 10⁻¹³ × 6.2 × 10⁻⁸/0.0248 = 1.2 × 10⁻¹⁹ M
[OH⁻] = Kw/[H₃O⁺] = 1.0 × 10⁻¹⁴/0.0248 = 4.0 × 10⁻¹³ M
(d) Explanation of Ka trend (1 point):
Each successive ionization becomes weaker because:
- Removing H⁺ from increasingly negative species requires more energy
- Electrostatic repulsion increases with negative charge
- The conjugate base becomes less stable with each ionization
Problem 5: Molecular Structure and Acidity (6 points)
Question: Consider the following carboxylic acids and their Ka values:
- CH₃COOH: Ka = 1.8 × 10⁻⁵
- CHCl₂COOH: Ka = 5.0 × 10⁻²
- CCl₃COOH: Ka = 0.20
(a) Rank these acids in order of increasing strength and explain the trend.
(b) Draw the Lewis structures for the conjugate bases of these acids.
(c) Explain how the inductive effect influences acid strength.
(d) Predict the relative Ka value for CH₂ClCOOH compared to the given acids.
Solution:
(a) Ranking by strength (2 points):
Increasing acid strength: CH₃COOH < CHCl₂COOH < CCl₃COOH
The trend follows the number of electronegative chlorine atoms. More chlorine atoms create stronger inductive effects, withdrawing electron density from the carboxyl group and stabilizing the conjugate base.
(b) Lewis structures (2 points):
All conjugate bases have the same basic structure with resonance:
[Image description: Acetate ion structure showing C-C-COO⁻ with resonance between C-O bonds]
The difference is in the substituents:
- CH₃COO⁻: methyl group (electron donating)
- CHCl₂COO⁻: dichloromethyl group (electron withdrawing)
- CCl₃COO⁻: trichloromethyl group (strongly electron withdrawing)
(c) Inductive effect explanation (1 point):
Electronegative chlorine atoms withdraw electron density through sigma bonds (inductive effect). This withdrawal:
- Destabilizes the acid (making H⁺ easier to remove)
- Stabilizes the conjugate base (by dispersing negative charge)
Both effects increase acid strength.
(d) Prediction for CH₂ClCOOH (1 point):
CH₂ClCOOH should have Ka between CH₃COOH and CHCl₂COOH. Expected Ka ≈ 1.4 × 10⁻³ (one chlorine provides moderate inductive effect, stronger than methyl but weaker than two chlorines).
Study Strategies and Exam Preparation Tips
Memory Techniques for Acids & Bases
1. Strong Acid Mnemonic: “HI, I’ll Have No Banana Split, Please”
- HI = HI (hydroiodic acid)
- I’ll = HCl (hydrochloric acid)
- Have = HBr (hydrobromic acid)
- No = HNO₃ (nitric acid)
- Banana = H₂SO₄ (sulfuric acid) – “Banana” for the yellow color
- Split = HClO₄ (perchloric acid) – “Split” for the explosive nature
- Please = Remember these are the six strong acids!
2. Strong Base Memory Device: “Loving Nobles Keep Rubies & Cesium, But Seek Calcium”
- Loving Nobles = LiOH, NaOH, KOH (Group 1 hydroxides)
- Rubies & Cesium = RbOH, CsOH (remaining Group 1)
- But Seek Calcium = Ba(OH)₂, Sr(OH)₂, Ca(OH)₂ (Group 2 hydroxides)
3. pH Scale Visualization
Create a mental ruler:
- 0-3: “Battery acid territory” (very acidic)
- 4-6: “Coffee and rain” (moderately acidic)
- 7: “Pure water” (neutral)
- 8-10: “Soap and antacids” (moderately basic)
- 11-14: “Household cleaners” (very basic)
Time Management Strategies
For Multiple Choice Questions:
- Budget 1.5 minutes per question
- Read the question stem first, then look at answer choices
- Eliminate obviously wrong answers immediately
- Use dimensional analysis to check your calculations
- Don’t spend more than 3 minutes on any single question
For Free Response Questions:
- Read all questions first and tackle easier ones initially
- Show all work clearly – partial credit is substantial
- Use proper chemical notation and significant figures
- Budget your time: ~23 minutes per long FR, ~9 minutes per short FR
- Leave space for calculations – cramped work leads to errors
Common Error Analysis
Top 5 Student Mistakes and How to Avoid Them:
1. Confusing Strong and Weak Acids/Bases
- Error: Treating all acids/bases the same way
- Solution: Memorize the short list of strong acids and bases; everything else is weak
- Red flag: If you’re using an equilibrium expression for HCl, you’re wrong!
2. ICE Table Setup Errors
- Error: Incorrect stoichiometry or signs in the change row
- Solution: Always check that your stoichiometry matches the balanced equation
- Practice tip: Write the balanced equation directly above your ICE table
3. Approximation Abuse
- Error: Using x << initial concentration when it’s not valid
- Solution: Check that x/initial concentration < 5% after solving
- Rule of thumb: If Ka/[initial] > 10⁻³, don’t approximate
4. Logarithm Calculation Errors
- Error: Calculator mistakes with log and -log functions
- Solution: Practice with your calculator; understand that pH = -log[H₃O⁺]
- Check: pH values should make chemical sense (very acidic = low pH)
5. Unit Confusion
- Error: Mixing molarity, molality, and moles
- Solution: Always write units in calculations and check dimensional consistency
- Buffer calculations: Make sure you’re using moles when applying Henderson-Hasselbalch
Effective Study Schedule
6 Weeks Before Exam:
- Week 1-2: Master fundamental concepts (acid-base theories, strong vs. weak)
- Week 3-4: Practice equilibrium calculations and ICE tables
- Week 5: Focus on buffers and titrations
- Week 6: Review and practice mixed problems
Daily Study Routine (45-60 minutes):
- Review notes (10 minutes): Previous day’s material
- New concept learning (20 minutes): Focus on one subtopic
- Practice problems (20 minutes): Mix of multiple choice and calculations
- Reflection (5 minutes): Identify concepts needing more work
Study Group Benefits:
- Explain concepts to others – teaches you better than just reading
- Work through different solution approaches – multiple pathways to understanding
- Practice verbal explanations – helps with free response clarity
Advanced Applications and Real-World Connections
Environmental Chemistry Applications
Acid Rain and pH
Acid rain, with pH values between 4.0-5.0, demonstrates acids and bases in environmental science. The primary culprits are:
Sulfuric Acid Formation:
SO₂(g) + H₂O(l) → H₂SO₃(aq) (sulfurous acid)
2H₂SO₃(aq) + O₂(g) → 2H₂SO₄(aq) (sulfuric acid)
Nitric Acid Formation:
NO₂(g) + H₂O(l) → HNO₃(aq) + HNO₂(aq)
Buffering in Natural Waters:
Natural water systems contain carbonate buffers that resist pH changes:
H₂CO₃/HCO₃⁻ and HCO₃⁻/CO₃²⁻ buffer systems help maintain aquatic ecosystem pH around 8.1-8.3.
Ocean Acidification:
Increased atmospheric CO₂ dissolves in seawater:
CO₂(g) + H₂O(l) ⇌ H₂CO₃(aq) ⇌ H⁺(aq) + HCO₃⁻(aq)
This process lowers ocean pH from 8.2 to 8.1 over the past century – seemingly small but representing a 30% increase in acidity due to the logarithmic scale.
Biological pH Regulation
Blood Buffer System:
Your blood maintains pH at 7.40 ± 0.05 through multiple buffer systems:
Primary Buffer: H₂CO₃/HCO₃⁻
H₂CO₃(aq) ⇌ H⁺(aq) + HCO₃⁻(aq)
The lungs control [H₂CO₃] by regulating CO₂:
H₂CO₃(aq) ⇌ CO₂(g) + H₂O(l)
The kidneys control [HCO₃⁻] by reabsorption and excretion.
Secondary Buffers:
- Phosphate buffer: H₂PO₄⁻/HPO₄²⁻ (pKa = 7.21)
- Protein buffers: Amino acid side chains with ionizable groups
Clinical Relevance:
- Acidosis: Blood pH < 7.35 (can be fatal below 7.0)
- Alkalosis: Blood pH > 7.45 (can be fatal above 7.8)
Industrial Applications
pH Control in Manufacturing:
Paper Industry:
- Pulping process: High pH (>12) with NaOH breaks down lignin
- Bleaching: Careful pH control prevents cellulose degradation
- Final product: Paper pH affects durability and printability
Food Industry:
- Cheese production: Lactic acid bacteria lower pH, enabling coagulation
- Wine making: pH affects color, stability, and microbial growth
- Soft drinks: Phosphoric acid (pH ~2.5) provides tartness and preservation
Water Treatment:
- Coagulation: Optimal pH (6.5-7.5) for aluminum sulfate effectiveness
- Disinfection: Chlorine effectiveness depends on pH (more effective at lower pH)
- Corrosion control: pH adjustment prevents pipe degradation
Pharmaceutical Chemistry
Drug Design and pH:
Many medications are weak acids or bases, and their effectiveness depends on pH:
Aspirin (Acetylsalicylic Acid):
- pKa = 3.5
- At stomach pH (1.5-2.0): predominantly molecular form, easily absorbed
- At blood pH (7.4): predominantly ionic form, less toxic to stomach lining
Local Anesthetics:
- Most are weak bases (pKa 8-9)
- Must be in molecular form to cross nerve membranes
- At physiological pH, equilibrium favors ionic form
- Infection lowers local pH, reducing anesthetic effectiveness
Buffer Systems in IV Solutions:
- Lactated Ringer’s: Contains lactate buffer for pH stability
- Phosphate buffers: Used in many injectable medications
- TRIS buffer: Common in biological research and some medications
FAQs
Q1: How do I know when to use the quadratic formula versus approximations in equilibrium problems?
Answer: Use the 5% rule as your guide. If Ka/[initial concentration] > 10⁻³, or if your approximation gives x > 5% of the initial concentration, use the quadratic formula.
Example: For 0.10 M acetic acid (Ka = 1.8 × 10⁻⁵):
Ka/[HA]₀ = 1.8 × 10⁻⁵/0.10 = 1.8 × 10⁻⁴ < 10⁻³
Approximation is likely valid, but always check your answer.
Q2: Why do some titration curves have multiple equivalence points?
Answer: Polyprotic acids (like H₂SO₄, H₃PO₄) can donate multiple protons, creating multiple equivalence points. Each equivalence point corresponds to the complete neutralization of one ionizable proton.
H₂SO₄ example:
- First equivalence point: H₂SO₄ → HSO₄⁻
- Second equivalence point: HSO₄⁻ → SO₄²⁻
The first equivalence point is usually much more pronounced because Ka1 >> Ka2.
Q3: How do I choose the right indicator for a titration?
Answer: Select an indicator whose transition range includes the equivalence point pH:
- Strong acid + Strong base: Any indicator (pH = 7 at equivalence point)
- Weak acid + Strong base: Use indicators like phenolphthalein (8.2-10.0)
- Strong acid + Weak base: Use indicators like methyl orange (3.1-4.4)
- Weak acid + Weak base: Avoid if possible; no sharp equivalence point
Q4: What’s the difference between endpoint and equivalence point?
Answer:
- Equivalence point: The theoretical point where moles of acid equal moles of base
- Endpoint: The observed point where the indicator changes color
In a well-designed titration, these should be very close, but they’re rarely identical.
Q5: Why can’t I just memorize all the Ka values?
Answer: While you should know common strong acids and bases, Ka values are typically provided on the AP exam. Focus on:
- Understanding the relationship between Ka and acid strength
- Recognizing structural factors that affect acidity
- Being comfortable with logarithmic calculations
Q6: How do buffer calculations change when I add strong acid or base?
Answer: Follow this systematic approach:
- Determine limiting reagent (added acid/base vs. buffer components)
- Calculate new moles after acid-base reaction
- Apply Henderson-Hasselbalch equation with new concentrations
- Check that buffer capacity isn’t exceeded
Q7: What happens to Kw at different temperatures?
Answer: Kw increases with temperature because water’s autoionization is endothermic:
- At 25°C: Kw = 1.0 × 10⁻¹⁴
- At 37°C: Kw = 2.4 × 10⁻¹⁴
- At 100°C: Kw = 1.0 × 10⁻¹²
Important: Neutral pH ≠ 7.00 at all temperatures!
Q8: Why do organic acids tend to be weaker than inorganic acids?
Answer: Several factors contribute:
- Electronegativity differences: C-H bonds vs. highly electronegative atoms
- Resonance stabilization: Limited in simple organic acids
- Inductive effects: Depend on substituents (electron-withdrawing groups increase acidity)
- Hybridization: sp³ carbon is less electronegative than sp² or sp carbon
Q9: How do I handle diprotic acid calculations?
Answer: Usually, the first ionization dominates if Ka1 >> Ka2 (difference of 10⁴ or more):
- Calculate [H₃O⁺] from first ionization only
- Use this [H₃O⁺] to find second ionization products
- For diprotic acids with similar Ka values, use systematic approach
Q10: What’s the most efficient way to study for the acids and bases unit?
Answer:
- Master the fundamentals: Strong vs. weak, equilibrium expressions
- Practice calculations daily: Start simple, build complexity
- Understand conceptually: Don’t just memorize formulas
- Connect to real applications: Makes concepts more memorable
- Use active recall: Test yourself without looking at notes
Conclusion: Your Path to Acids & Bases Mastery
Congratulations! You’ve just completed a comprehensive journey through AP Chemistry Unit 8: Acids & Bases. This unit, representing 11-15% of your AP exam, is now within your grasp. Let’s solidify what you’ve accomplished and outline your next steps for continued success.
What You’ve Mastered
Conceptual Understanding:
- The three major acid-base theories and their applications
- The fundamental difference between strong and weak acids and bases
- How molecular structure influences acid and base strength
- The intricate relationships between pH, pOH, Ka, Kb, and pKa
Problem-Solving Skills:
- pH and pOH calculations for both strong and weak systems
- ICE table methodology for equilibrium problems
- Buffer system analysis using Henderson-Hasselbalch equation
- Titration curve interpretation and indicator selection
- Multi-step calculations involving polyprotic acids
Real-World Applications:
- Environmental chemistry connections (acid rain, ocean acidification)
- Biological pH regulation and buffer systems
- Industrial applications in manufacturing and pharmaceuticals
- The critical role of pH in everyday life
Key Success Strategies Reinforced
1. Pattern Recognition: You’ve learned that acids and bases follow predictable patterns. Strong acids and bases behave similarly, weak systems use equilibrium principles, and buffers resist pH changes through Le Châtelier’s principle.
2. Systematic Approach: Whether solving pH problems or analyzing titration curves, following consistent steps reduces errors and builds confidence.
3. Conceptual Foundation: Understanding why acids and bases behave as they do makes memorization unnecessary and problem-solving intuitive.
Advanced Study Recommendations
For Students Aiming for 5s:
- Explore the connections between acids-bases and thermodynamics
- Study advanced buffer systems and their applications
- Investigate the molecular orbital theory explanations for acid-base strength
- Practice challenging multi-step problems combining several units
For Students Targeting 4s:
- Focus on mastering the core calculations with consistent accuracy
- Strengthen your understanding of titration curves and their interpretations
- Practice explaining your reasoning clearly for free response questions
- Build confidence with a variety of buffer problems
For Students Working Toward 3s:
- Ensure solid understanding of strong acid/base calculations
- Master the basic Henderson-Hasselbalch applications
- Practice identifying acid-base pairs and conjugate relationships
- Focus on the most common problem types from past exams
Motivation for Your Journey Ahead
Remember, mastering acids and bases isn’t just about passing an exam – you’re developing analytical thinking skills that will serve you throughout your scientific career. Every time you:
- Calculate pH, you’re applying logarithmic thinking
- Analyze a buffer system, you’re using equilibrium principles
- Interpret a titration curve, you’re connecting molecular behavior to macroscopic observations
- Explain acid strength trends, you’re thinking about electronic structure and molecular stability
These skills transfer directly to advanced chemistry courses, medical school preparation, environmental science, and countless other fields where quantitative analysis matters.
Final Encouragement
The journey through AP Chemistry Unit 8 challenges every student, but you’ve equipped yourself with the tools for success. You understand the theory, you’ve practiced the calculations, and you’ve connected the concepts to real-world applications.
Trust in your preparation, approach problems systematically, and remember that every successful chemist once stood where you stand now – ready to demonstrate their mastery of acids and bases.
Your AP Chemistry success story continues beyond this unit. Use the confidence and skills you’ve built here as stepping stones to excellence in the remaining units and ultimately, your best possible exam performance.
The chemistry community welcomes your growing expertise. Now go show the AP exam what you’ve learned!
This comprehensive guide represents the collective wisdom of successful AP Chemistry students and educators. Bookmark it, share it with classmates, and return to it whenever you need clarification or extra practice. Your success in AP Chemistry Unit 8: Acids & Bases starts with understanding – and you’ve just achieved that understanding.
About Solvefy AI: Your trusted partner in AP Chemistry success, providing comprehensive study guides, practice problems, and expert insights to help students achieve their highest potential on the AP Chemistry exam.
Also Read –

1 thought on “AP Chemistry Unit 8: Acids & Bases – Complete Mastery Guide for 2025 Exam Success”