The Chemistry Around Us
Have you ever wondered why lemon juice makes your mouth pucker, or why soap feels slippery between your fingers? The answer lies in the fascinating world of acids, bases, and salts – Topic 7 of your IGCSE Chemistry syllabus. This isn’t just textbook theory; it’s the chemistry that shapes our daily lives, from the food we eat to the cleaning products we use.
Whether you’re struggling to understand pH values or trying to master neutralization equations, this comprehensive guide will transform your understanding of acids, bases, and salts. By the end, you’ll not only ace your exams but also see chemistry everywhere around you!
What Are Acids? Breaking Down the Basics
Definition and Properties
An acid is a substance that releases hydrogen ions (H⁺) when dissolved in water. Think of acids as hydrogen ion donors – they’re generous with their H⁺ ions!
Key Properties of Acids:
- Taste sour (though NEVER taste chemicals in the lab!)
- Turn blue litmus paper red
- React with metals to produce hydrogen gas
- React with carbonates to produce carbon dioxide
- Conduct electricity (they’re electrolytes)
- Have pH values less than 7
Common Examples of Acids
Strong Acids (completely ionize in water):
- Hydrochloric acid (HCl) – found in stomach acid
- Sulfuric acid (H₂SO₄) – used in car batteries
- Nitric acid (HNO₃) – used in fertilizers
Weak Acids (partially ionize in water):
- Ethanoic acid (CH₃COOH) – found in vinegar
- Citric acid – found in citrus fruits
- Carbonic acid (H₂CO₃) – found in fizzy drinks
Memory Tip
Remember “ACIDS ARE SOUR” – Always Conduct electricity, Ionize in water, Donate H⁺ ions, Sour taste, And Red litmus, Electrolytes, Strong or weak, Oxidize metals, Under pH 7, Reactive.
Understanding Bases: The Other Side of the Coin
Definition and Properties
A base is a substance that releases hydroxide ions (OH⁻) when dissolved in water, or accepts hydrogen ions (H⁺). Bases are the opposite of acids – they’re hydrogen ion acceptors!
Key Properties of Bases:
- Taste bitter and feel slippery
- Turn red litmus paper blue
- React with acids to form salts and water
- Conduct electricity
- Have pH values greater than 7
Common Examples of Bases
Strong Bases (completely ionize in water):
- Sodium hydroxide (NaOH) – found in drain cleaners
- Potassium hydroxide (KOH) – used in soap making
- Calcium hydroxide (Ca(OH)₂) – lime water
Weak Bases (partially ionize in water):
- Ammonia (NH₃) – found in household cleaners
- Sodium hydrogen carbonate (NaHCO₃) – baking soda
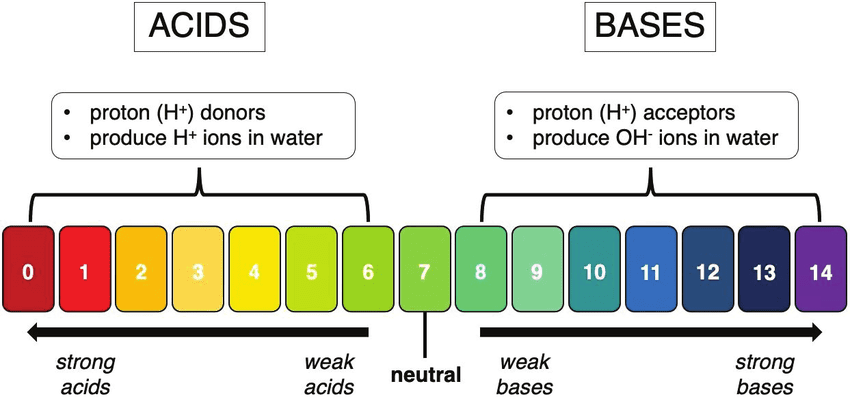
The pH Scale: Measuring Acidity and Alkalinity
Understanding pH
The pH scale ranges from 0 to 14 and measures how acidic or basic a solution is. pH stands for “potential of Hydrogen” or “power of Hydrogen.”
pH Scale Breakdown:
- pH 0-6: Acidic (lower numbers = more acidic)
- pH 7: Neutral (pure water)
- pH 8-14: Basic/Alkaline (higher numbers = more basic)
Real-Life pH Examples
| Substance | Approximate pH | Classification |
|---|---|---|
| Battery acid | 0-1 | Very acidic |
| Lemon juice | 2 | Acidic |
| Coffee | 5 | Weakly acidic |
| Pure water | 7 | Neutral |
| Baking soda | 9 | Basic |
| Household ammonia | 11 | Very basic |
| Bleach | 13 | Very basic |
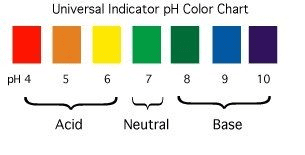
Calculating pH
For strong acids and bases, you can calculate pH using:
- pH = -log[H⁺]
- pOH = -log[OH⁻]
- pH + pOH = 14 (at 25°C)
Indicators: Nature’s pH Detectors
What Are Indicators?
Indicators are substances that change color depending on whether they’re in acidic or basic solutions. They’re like chemical chameleons!
Types of Indicators
Litmus:
- Red litmus → Blue in basic solutions
- Blue litmus → Red in acidic solutions
- Purple litmus → stays purple in neutral solutions
Universal Indicator:
- Shows different colors across the entire pH range
- More precise than simple litmus
- Red (very acidic) → Orange → Yellow → Green (neutral) → Blue → Purple (very basic)
Methyl Orange:
- Red in acidic solutions (pH < 4.4)
- Yellow in basic solutions (pH > 6.2)
Phenolphthalein:
- Colorless in acidic solutions
- Pink/magenta in basic solutions (pH > 8.2)
Memory Tip for Litmus
“Red acid, Blue base” – remember this simple rhyme and you’ll never mix them up!
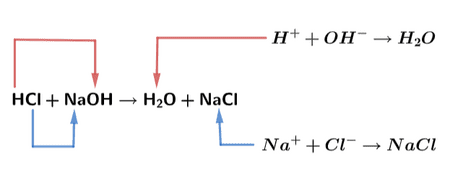
Neutralization Reactions: When Acids Meet Bases
The Basic Equation
When acids and bases react, they undergo neutralization, producing salt and water:
Acid + Base → Salt + Water
This is one of the most important reactions in chemistry!
Step-by-Step Neutralization Process
- Mixing: Acid and base solutions are combined
- Ion Exchange: H⁺ ions from acid react with OH⁻ ions from base
- Water Formation: H⁺ + OH⁻ → H₂O
- Salt Formation: Remaining ions combine to form salt
- Heat Release: Energy is released (exothermic reaction)
Examples of Neutralization Reactions
Hydrochloric acid + Sodium hydroxide:
HCl + NaOH → NaCl + H₂O
Sulfuric acid + Potassium hydroxide:
H₂SO₄ + 2KOH → K₂SO₄ + 2H₂O
Nitric acid + Ammonia:
HNO₃ + NH₃ → NH₄NO₃
Salts: The Products of Neutralization
What Are Salts?
Salts are ionic compounds formed when the hydrogen ion of an acid is replaced by a metal ion or ammonium ion. They’re not just table salt (NaCl)!
Types of Salts
Normal Salts:
- Formed by complete neutralization
- No acidic or basic properties
- Examples: NaCl, KBr, CaSO₄
Acid Salts:
- Formed by partial neutralization of polyprotic acids
- Still contain replaceable hydrogen
- Examples: NaHSO₄, NaHCO₃
Basic Salts:
- Contain hydroxide ions
- Examples: Mg(OH)Cl, Zn(OH)Cl
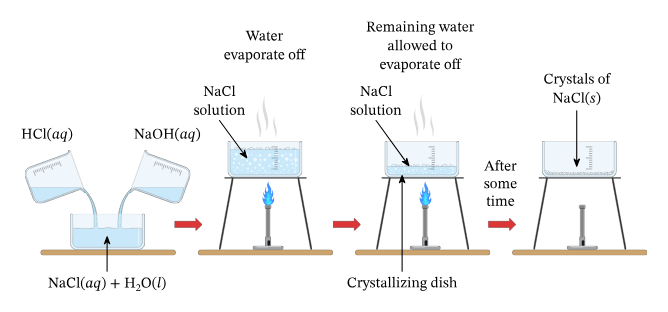
Salt Preparation Methods
1. Acid + Metal:
- Mg + 2HCl → MgCl₂ + H₂
- Only works with metals above hydrogen in reactivity series
2. Acid + Metal Oxide:
- CuO + H₂SO₄ → CuSO₄ + H₂O
- Works for most metal oxides
3. Acid + Metal Carbonate:
- CaCO₃ + 2HCl → CaCl₂ + H₂O + CO₂
- Produces carbon dioxide gas
4. Acid + Base:
- HCl + NaOH → NaCl + H₂O
- The classic neutralization reaction
Naming Salts
The name of a salt comes from:
- Metal part: From the base or metal
- Non-metal part: From the acid
Acid → Salt ending:
- Hydrochloric acid → Chloride
- Sulfuric acid → Sulfate
- Nitric acid → Nitrate
- Ethanoic acid → Ethanoate
- Carbonic acid → Carbonate
Practical Applications in Everyday Life
In Your Kitchen
- Baking: Sodium hydrogen carbonate (baking soda) releases CO₂ when heated
- Preservation: Vinegar (ethanoic acid) preserves food
- Cleaning: Lemon juice (citric acid) removes limescale
In Medicine
- Antacids: Neutralize excess stomach acid
- Aspirin: Acetylsalicylic acid for pain relief
- IV fluids: Carefully pH-balanced solutions
In Industry
- Metal extraction: Acids dissolve metal ores
- Fertilizers: Ammonium salts provide nitrogen for plants
- Soap making: Saponification uses strong bases
In Environmental Science
- Acid rain: Environmental problem caused by sulfur and nitrogen oxides
- Ocean pH: Climate change affects ocean acidity
- Soil treatment: Lime reduces soil acidity
Test Yourself: Quick Check Questions
- What happens to blue litmus paper in acidic solution?
- Name three strong acids commonly used in laboratories.
- What is the pH of a neutral solution at 25°C?
- Complete this equation: HNO₃ + KOH → ? + ?
- Which indicator is colorless in acidic solution but pink in basic solution?
Answers:
- Turns red
- HCl, H₂SO₄, HNO₃
- pH = 7
- KNO₃ + H₂O
- Phenolphthalein
Common Mistakes to Avoid
During Experiments
- Never add water to concentrated acid – always add acid to water
- Don’t taste chemicals – even if they’re food acids in the lab
- Wear safety equipment – acids and bases can cause burns
In Calculations
- pH + pOH = 14 only at 25°C
- Remember significant figures in pH calculations
- Check your ionic equations for charge balance
In Exams
- Learn the indicators properly – don’t confuse litmus colors
- Balance equations correctly – count atoms on both sides
- Use proper chemical names – not common names
Key Formulas and Equations Box
Essential Equations:
- General neutralization: Acid + Base → Salt + Water
- pH calculation: pH = -log[H⁺]
- pH and pOH relationship: pH + pOH = 14
- Water ionization: H₂O ⇌ H⁺ + OH⁻
Important Reactions:
- Metal + Acid → Salt + Hydrogen
- Metal Oxide + Acid → Salt + Water
- Metal Carbonate + Acid → Salt + Water + Carbon Dioxide
- Ammonia + Acid → Ammonium Salt
Quick Revision Notes
Acids Quick Facts
✓ Release H⁺ ions in water
✓ pH < 7
✓ Turn blue litmus red
✓ React with metals, carbonates, and bases
✓ Conduct electricity
Bases Quick Facts
✓ Release OH⁻ ions or accept H⁺ ions
✓ pH > 7
✓ Turn red litmus blue
✓ Feel slippery and taste bitter
✓ React with acids in neutralization
Salts Quick Facts
✓ Ionic compounds from acid-base reactions
✓ Named from acid and base used
✓ Can be prepared by four main methods
✓ Important in industry and daily life
pH Scale Memory
✓ 0-6: Acidic (red on universal indicator)
✓ 7: Neutral (green on universal indicator)
✓ 8-14: Basic (blue/purple on universal indicator)
Important Exam Questions to Practice
Structured Questions
- Describe and explain the difference between strong and weak acids, giving examples of each. (6 marks)
- A student wants to prepare crystals of copper sulfate from copper oxide.
a) Write the chemical equation for this reaction. (2 marks)
b) Describe the method the student should use. (4 marks)
c) How could the student test that the reaction is complete? (2 marks) - Explain why universal indicator is more useful than litmus for measuring pH. Include the color changes you would expect to see. (4 marks)
Multiple Choice Practice
- Which of these solutions has the highest concentration of H⁺ ions?
- A) pH 2 B) pH 5 C) pH 9 D) pH 12
- What is formed when zinc reacts with hydrochloric acid?
- A) Zinc chloride only
- B) Zinc chloride and oxygen
- C) Zinc chloride and hydrogen
- D) Zinc hydroxide and hydrogen
Extended Response
- “Acids and bases are important in many aspects of daily life.” Discuss this statement, giving specific examples of acids and bases encountered in everyday situations and explaining their uses. (8 marks)
Study Tips for Success
Before the Exam
- Make flashcards for acid/base properties and indicators
- Practice equation balancing daily for 10 minutes
- Draw pH scale from memory with examples
- Memorize strong acids and bases – they appear frequently
During Practical Work
- Observe color changes carefully – they’re often exam questions
- Record pH readings precisely – note decimal places
- Practice using different indicators – know when to use each one
Memory Techniques
- Create acronyms for lists (like strong acids: “His Sulky Nose” for HCl, H₂SO₄, HNO₃)
- Use analogies – think of acids as “ion donors” being generous
- Visual learning – draw out molecular diagrams
- Connect to real life – link concepts to everyday examples
Looking Ahead: What’s Next?
Congratulations! You’ve mastered one of the most practical topics in IGCSE Chemistry. Understanding acids, bases, and salts opens doors to:
Related Topics to Explore
- Redox reactions – how acids participate in electron transfer
- Organic chemistry – carboxylic acids and their properties
- Environmental chemistry – acid rain and its effects
- Quantitative analysis – using acid-base titrations
Further Study Opportunities
- A-Level Chemistry – deeper understanding of acid-base theories
- Biochemistry – pH control in biological systems
- Environmental Science – impact of acids and bases on ecosystems
- Industrial Chemistry – large-scale acid and base production
Career Connections
This knowledge is valuable for careers in:
- Medicine and healthcare
- Environmental science
- Food science and nutrition
- Chemical engineering
- Pharmaceutical research
Final Encouragement
Remember, chemistry isn’t just about memorizing facts and formulas – it’s about understanding the world around you. Every time you see fizzy drinks bubble, watch antacids work, or notice how soap cleans, you’re seeing the principles from this topic in action.
Don’t worry if some concepts seem challenging at first. Even professional chemists had to start somewhere! The key is consistent practice, asking questions when you’re stuck, and connecting new knowledge to what you already understand.
Your IGCSE Chemistry journey is preparing you for exciting possibilities. Whether you continue with A-Levels, pursue a science career, or simply become a more informed citizen, understanding acids, bases, and salts gives you insights into countless processes that shape our world.
Keep experimenting, keep questioning, and most importantly, keep that curiosity alive. Chemistry is everywhere – you just need to know how to look for it!
Good luck with your studies, and remember: you’ve got this!
Recommended –


1 thought on “IGCSE (Cambridge) Chemistry Topic 7: Acids, Bases and Salts | Your Complete Study Guide”