Welcome to the most comprehensive guide for AP Chemistry Unit 1: Atomic Structure and Properties. As we advance into 2025, understanding atomic structure has become more critical than ever, with applications spanning from quantum computing to advanced materials science. Whether you’re a student in New York, Mumbai, São Paulo, or Sydney, this guide will provide you with the foundational knowledge needed to excel in AP Chemistry.
Why Unit 1 Matters More Than Ever
In today’s rapidly evolving scientific landscape, atomic structure forms the bedrock of everything from nanotechnology to pharmaceutical development. The principles you’ll learn in Unit 1 aren’t just academic concepts-they’re the tools that scientists use to design new materials, develop life-saving drugs, and push the boundaries of technology.
The Challenge Students Face
Many AP Chemistry students struggle with Unit 1 because it requires a fundamental shift in thinking. You’re moving from the macroscopic world you can see and touch to the microscopic realm of atoms and electrons. This transition can be overwhelming, especially when you’re dealing with concepts like:
- Quantum mechanical models that defy everyday intuition
- Mathematical relationships between microscopic and macroscopic quantities
- Abstract concepts like electron probability distributions
- Complex periodic trends that seem to have multiple exceptions
What This Guide Offers
This comprehensive resource addresses these challenges head-on. You’ll find:
Crystal-clear explanations of complex concepts using real-world analogies
Step-by-step problem-solving strategies with worked examples
Visual learning tools including diagrams, charts, and interactive elements
International perspective with examples from global universities and research
2025-updated content reflecting the latest AP Chemistry curriculum changes
Exam-focused preparation with authentic AP-style questions and solutions
Unit 1 by the Numbers
- Exam Weightage: 7-9% of your total AP Chemistry score
- Recommended Study Time: 2-3 weeks of focused preparation
- Topics Covered: 8 essential concepts that build upon each other
- Mathematical Skills: Dimensional analysis, proportional reasoning, logarithms
- Laboratory Connections: Mass spectrometry, photoelectron spectroscopy
Your Success Roadmap
This guide follows a proven learning progression:
- Foundation Building: Core concepts and historical context
- Skill Development: Problem-solving techniques and mathematical applications
- Mastery Achievement: Advanced applications and exam preparation
- Beyond AP: Connections to college chemistry and real-world applications
By the end of this guide, you’ll not only be prepared for the AP Chemistry exam but also equipped with a deep understanding of atomic structure that will serve you throughout your scientific journey.
Unit 1 At-a-Glance
| Aspect | Details |
|---|---|
| Exam Weight | 7-9% of total AP Chemistry score |
| Study Duration | 2-3 weeks recommended |
| Topics | 8 interconnected concepts |
| Key Skills | Dimensional analysis, trend prediction, data interpretation |
| Laboratory Focus | Mass spectrometry, PES analysis |
| Mathematical Tools | Scientific notation, proportional reasoning, Coulomb’s law |
Eight Essential Topics
- Topic 1.1: Moles and Molar Mass
- Topic 1.2: Mass Spectra of Elements
- Topic 1.3: Elemental Composition of Pure Substances
- Topic 1.4: Composition of Mixtures
- Topic 1.5: Atomic Structure and Electron Configuration
- Topic 1.6: Photoelectron Spectroscopy (PES)
- Topic 1.7: Periodic Trends
- Topic 1.8: Valence Electrons and Ionic Compounds
Essential Formulas Card
| Formula | Application | Units |
|---|---|---|
| n = m/M | Moles calculation | mol |
| F ∝ q₁q₂/r² | Coulomb’s law | N (Newtons) |
| Average Atomic Mass | Weighted isotope average | amu |
| % Composition | (mass of element/total mass) × 100 | % |
| Empirical Formula | Simplest whole number ratio | – |
Key Terms Preview
- Mole (mol): 6.022 × 10²³ particles
- Isotopes: Same element, different mass numbers
- Electron Configuration: Electron arrangement in atoms
- Ionization Energy: Energy to remove an electron
- Electronegativity: Attraction for bonding electrons
- Effective Nuclear Charge: Net positive charge felt by electrons
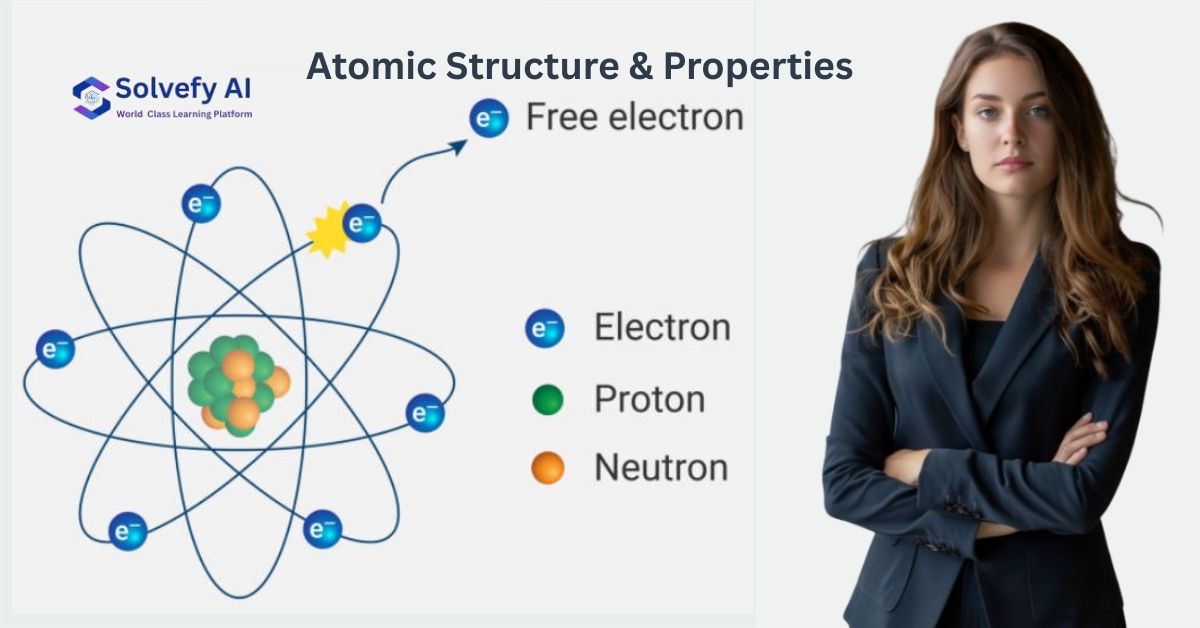
The Atomic Theory Revolution
The journey to understanding atomic structure represents one of humanity’s greatest intellectual achievements. From John Dalton’s simple atomic theory in 1803 to the sophisticated quantum mechanical model we use today, each breakthrough has revolutionized our understanding of matter.
Historical Timeline:
- 1803: Dalton’s Atomic Theory – atoms as indivisible particles
- 1897: Thomson’s “Plum Pudding” model – discovery of electrons
- 1911: Rutherford’s Nuclear Model – concentrated positive nucleus
- 1913: Bohr’s Planetary Model – quantized electron orbits
- 1926: Quantum Mechanical Model – electron probability distributions
Why This History Matters
Understanding this progression helps you appreciate why certain concepts might seem counterintuitive. The quantum mechanical model, which forms the basis of modern chemistry, required scientists to abandon classical physics intuitions about particle behavior.
Modern Atomic Model Components:
- Nucleus: Contains protons and neutrons (99.9% of atom’s mass)
- Electron Cloud: Probability distribution of electron locations
- Energy Levels: Quantized electron energy states
- Orbitals: Three-dimensional regions of electron probability
Topic 1.1: The Mole Concept Mastery
The mole concept is arguably the most important mathematical tool in chemistry. It serves as the bridge between the microscopic world of atoms and molecules and the macroscopic world of grams and liters that we can measure in the laboratory.
Core Concept: Think of the mole as chemistry’s “dozen.” Just as a dozen always means 12 items (whether eggs, donuts, or pencils), a mole always means 6.022 × 10²³ particles (whether atoms, molecules, or ions).
Avogadro’s Number: 6.022 × 10²³ mol⁻¹
This enormous number represents the number of carbon-12 atoms in exactly 12 grams of carbon-12. To put this in perspective:
- If you could count one atom per second, it would take 1.9 × 10¹⁶ years to count one mole of atoms
- One mole of rice grains would cover the Earth’s surface to a depth of about 75 meters
The Molar Mass Connection
The genius of the mole concept lies in its connection between atomic mass units (amu) and grams:
- 1 carbon-12 atom has a mass of 12 amu
- 1 mole of carbon-12 atoms has a mass of 12 grams
- Therefore: atomic mass in amu = molar mass in g/mol
Practical Applications:
- Converting between mass and moles:
n = m/M
where: n = moles, m = mass (g), M = molar mass (g/mol)- Converting between moles and particles:
Number of particles = n × Nₐ
where: n = moles, Nₐ = Avogadro's numberCommon Student Mistakes and How to Avoid Them:
Mistake: Confusing molar mass with atomic mass
Solution: Remember that molar mass has units (g/mol), atomic mass doesn’t
Mistake: Forgetting to use Avogadro’s number when converting moles to particles
Solution: Always write out the conversion factor: 1 mol = 6.022 × 10²³ particles
Mistake: Using the wrong molar mass for compounds
Solution: Always add up the atomic masses of all atoms in the formula
Practice Problem:
How many oxygen atoms are in 2.5 g of calcium carbonate (CaCO₃)?
Solution:
- Find molar mass of CaCO₃: 40.08 + 12.01 + 3(16.00) = 100.09 g/mol
- Convert mass to moles: n = 2.5 g ÷ 100.09 g/mol = 0.0250 mol CaCO₃
- Find moles of O atoms: 0.0250 mol CaCO₃ × 3 mol O/mol CaCO₃ = 0.0750 mol O
- Convert to atoms: 0.0750 mol × 6.022 × 10²³ atoms/mol = 4.52 × 10²² atoms
Topic 1.1: Moles and Molar Mass
Learning Objective: Calculate quantities of a substance or component of a substance in moles and relate quantities in moles to masses in grams using dimensional analysis.
Essential Knowledge Points:
- The mole allows a chemist to count particles by weighing
- One mole contains Avogadro’s number (6.022 × 10²³) of particles
- The mass of one mole of a substance (molar mass) equals its atomic/molecular mass in grams
- Dimensional analysis is the key problem-solving tool
- Set up conversion factors to cancel unwanted units
- Always check that your final answer has the correct units
- Use the factor-label method for complex conversions
Problem-Solving Strategy:
Step 1: Identify what you’re given and what you need to find
Step 2: Write down relevant formulas and conversion factors
Step 3: Set up the dimensional analysis
Step 4: Calculate and check units
Step 5: Verify that your answer makes sense
Practice Examples:
Example 1: How many moles are in 45.6 g of glucose (C₆H₁₂O₆)?
Solution:
- Molar mass of C₆H₁₂O₆ = 6(12.01) + 12(1.008) + 6(16.00) = 180.16 g/mol
- n = 45.6 g ÷ 180.16 g/mol = 0.253 mol glucose
Example 2: What is the mass of 2.45 × 10²³ molecules of water?
Solution:
- Convert molecules to moles: (2.45 × 10²³ molecules) ÷ (6.022 × 10²³ molecules/mol) = 0.407 mol
- Molar mass of H₂O = 2(1.008) + 16.00 = 18.016 g/mol
- Mass = 0.407 mol × 18.016 g/mol = 7.33 g
Example 3: How many carbon atoms are in 25.0 g of ethanol (C₂H₅OH)?
Solution:
- Molar mass of C₂H₅OH = 2(12.01) + 6(1.008) + 16.00 = 46.068 g/mol
- Moles of ethanol = 25.0 g ÷ 46.068 g/mol = 0.543 mol
- Moles of C atoms = 0.543 mol ethanol × 2 mol C/mol ethanol = 1.086 mol C
- Number of C atoms = 1.086 mol × 6.022 × 10²³ atoms/mol = 6.54 × 10²³ atoms
AP Exam Tips:
- Pay attention to significant figures in your calculations
- Show all work clearly, even on multiple choice questions
- Use proper scientific notation for very large or small numbers
- Double-check that you’ve answered the question being asked
Topic 1.2: Mass Spectra of Elements
Learning Objective: Explain the quantitative relationship between the elemental composition by mass and the empirical formula of a pure substance.
Understanding Mass Spectrometry:
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio (m/z) of ions. For atoms (which typically have a +1 charge), this directly gives us the mass of different isotopes.
How Mass Spectrometry Works:
- Ionization: Atoms are ionized (usually by electron bombardment)
- Acceleration: Ions are accelerated through an electric field
- Separation: Ions are separated based on their mass-to-charge ratio
- Detection: The abundance of each mass is recorded
Isotope Identification Techniques:
When you look at a mass spectrum:
- x-axis: Mass-to-charge ratio (m/z), which equals mass for +1 ions
- y-axis: Relative abundance (usually as a percentage)
- Peaks: Each peak represents a different isotope
Average Atomic Mass Calculations:
The average atomic mass is calculated using the weighted average formula:
Average atomic mass = Σ(isotope mass × fractional abundance)Real MS Data Interpretation Example:
Consider the mass spectrum of chlorine:
- Peak at m/z = 35: 75.8% abundance (³⁵Cl)
- Peak at m/z = 37: 24.2% abundance (³⁷Cl)
Average atomic mass = (35 × 0.758) + (37 × 0.242) = 26.53 + 8.954 = 35.48 amu
Practice Problems:
Problem 1: Boron has two isotopes: ¹⁰B (19.9% abundance) and ¹¹B (80.1% abundance). Calculate the average atomic mass.
Solution:
Average atomic mass = (10 × 0.199) + (11 × 0.801) = 1.99 + 8.811 = 10.80 amu
Problem 2: An element has three isotopes with the following data:
- Isotope A: mass = 24 amu, abundance = 78.9%
- Isotope B: mass = 25 amu, abundance = 10.0%
- Isotope C: mass = 26 amu, abundance = 11.1%
What is the average atomic mass?
Solution:
Average atomic mass = (24 × 0.789) + (25 × 0.100) + (26 × 0.111)
= 18.936 + 2.500 + 2.886 = 24.32 amu
Topic 1.3: Elemental Composition of Pure Substances
Learning Objective: Explain the quantitative relationship between the elemental composition by mass and the composition of substances in a mixture.
Empirical vs Molecular Formulas:
- Empirical Formula: Simplest whole-number ratio of atoms in a compound
- Molecular Formula: Actual number of atoms in a molecule
- Relationship: Molecular formula = (Empirical formula) × n, where n is a whole number
Law of Definite Proportions:
Joseph Proust’s law states that a chemical compound always contains exactly the same proportion of elements by mass. This means:
- Pure water is always 88.8% oxygen and 11.2% hydrogen by mass
- This ratio is constant regardless of the source of the water
Combustion Analysis:
This is a common experimental method for determining empirical formulas of organic compounds:
- Burn the compound in excess oxygen
- Measure the masses of CO₂ and H₂O produced
- Calculate moles of C and H from the products
- Determine the empirical formula from the mole ratio
Step-by-Step Problem Solving:
Example: A 2.50 g sample of a compound containing only C, H, and O produces 3.66 g CO₂ and 1.50 g H₂O upon combustion. Find the empirical formula.
Solution:
Step 1: Find moles of C and H from the products
- Moles of C = moles of CO₂ = 3.66 g ÷ 44.01 g/mol = 0.0831 mol C
- Moles of H = 2 × moles of H₂O = 2 × (1.50 g ÷ 18.02 g/mol) = 0.167 mol H
Step 2: Find mass of C and H in the original sample
- Mass of C = 0.0831 mol × 12.01 g/mol = 0.998 g C
- Mass of H = 0.167 mol × 1.008 g/mol = 0.168 g H
Step 3: Find mass and moles of O by difference
- Mass of O = 2.50 g – 0.998 g – 0.168 g = 1.33 g O
- Moles of O = 1.33 g ÷ 16.00 g/mol = 0.0831 mol O
Step 4: Find the simplest ratio
- C: 0.0831 ÷ 0.0831 = 1.00
- H: 0.167 ÷ 0.0831 = 2.01 ≈ 2
- O: 0.0831 ÷ 0.0831 = 1.00
Empirical formula: CH₂O
Topic 1.4: Composition of Mixtures
Learning Objective: Explain the quantitative relationship between the elemental composition by mass and the composition of substances in a mixture.
Pure Substances vs Mixtures:
- Pure Substances: Fixed composition, consistent properties
- Elements (O₂, Fe, C)
- Compounds (H₂O, NaCl, C₆H₁₂O₆)
- Mixtures: Variable composition, properties depend on composition
- Homogeneous (solutions): uniform throughout
- Heterogeneous: visibly different phases
Elemental Analysis Applications:
- Quality Control: Ensuring products meet specifications
- Environmental Monitoring: Detecting pollutants in air, water, soil
- Forensic Science: Analyzing evidence samples
- Pharmaceutical Testing: Verifying drug purity and composition
Purity Determination Methods:
Method 1: Mass Percent Calculation
Mass % = (mass of component / total mass) × 100%Method 2: Mole Fraction
Mole fraction (χ) = moles of component / total molesPractice Problem: A mixture contains 15.0 g of NaCl and 35.0 g of water. Calculate:
a) Mass percent of each component
b) Mole fraction of each component
Solution:
a) Mass percentages:
- Total mass = 15.0 g + 35.0 g = 50.0 g
- Mass % NaCl = (15.0 g / 50.0 g) × 100% = 30.0%
- Mass % H₂O = (35.0 g / 50.0 g) × 100% = 70.0%
b) Mole fractions:
- Moles NaCl = 15.0 g ÷ 58.44 g/mol = 0.257 mol
- Moles H₂O = 35.0 g ÷ 18.02 g/mol = 1.94 mol
- Total moles = 0.257 + 1.94 = 2.20 mol
- χ(NaCl) = 0.257 mol ÷ 2.20 mol = 0.117
- χ(H₂O) = 1.94 mol ÷ 2.20 mol = 0.883
Topic 1.5: Atomic Structure and Electron Configuration
Learning Objective: Represent the electron configuration of an element or ions of an element using the Aufbau principle.
Quantum Mechanical Model:
The modern model describes electrons not as particles in defined orbits, but as waves with probability distributions. Key concepts:
- Wave-Particle Duality: Electrons exhibit both wave and particle properties
- Uncertainty Principle: Cannot know both position and momentum precisely
- Quantum Numbers: Four numbers that describe each electron’s state
Aufbau Principle Application:
The Aufbau principle states that electrons fill orbitals in order of increasing energy. The order is:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p…
Memory Device: Use the diagonal rule or remember: “Silly People Don’t Fear Difficult Problems”
Core vs Valence Electrons:
- Core Electrons: Inner electrons that don’t participate in bonding
- Valence Electrons: Outermost electrons that determine chemical properties
- Valence Shell: The highest principal energy level containing electrons
Coulomb’s Law: F ∝ q₁q₂/r²:
This fundamental law explains many periodic trends:
- Force increases with larger charges (q₁, q₂)
- Force decreases with greater distance (r)
- Applications: ionization energy, atomic radius, electron-electron repulsion
Electron Configuration Rules:
- Aufbau Principle: Fill lowest energy orbitals first
- Pauli Exclusion Principle: Maximum 2 electrons per orbital, opposite spins
- Hund’s Rule: Fill orbitals singly before pairing electrons
Examples:
- Carbon (6 electrons): 1s² 2s² 2p² or [He] 2s² 2p²
- Iron (26 electrons): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶ or [Ar] 4s² 3d⁶
- Bromine (35 electrons): [Ar] 4s² 3d¹⁰ 4p⁵
Ion Configuration:
For cations (positive ions): Remove electrons from highest energy orbitals first
- Fe²⁺: [Ar] 3d⁶ (remove 4s electrons first)
- Cu⁺: [Ar] 3d¹⁰ (remove 4s electron)
For anions (negative ions): Add electrons to the next available orbital
- Cl⁻: [Ar] or [Ne] 3s² 3p⁶
- O²⁻: [Ne] or 1s² 2s² 2p⁶
Topic 1.6: Photoelectron Spectroscopy (PES)
Learning Objective: Explain the relationship between the photoelectron spectrum of an atom or ion and the electron configuration and binding energy of electrons.
PES Theory and Applications:
Photoelectron spectroscopy measures the energy required to remove electrons from atoms. When high-energy photons strike a sample:
- Energy Transfer: Photon energy transfers to electrons
- Electron Ejection: Electrons with sufficient energy are ejected
- Energy Measurement: Kinetic energy of ejected electrons is measured
- Binding Energy Calculation: E(photon) = E(binding) + E(kinetic)
Peak Analysis Techniques:
In a PES spectrum:
- x-axis: Binding energy (MJ/mol) – energy needed to remove electron
- y-axis: Number of electrons (intensity)
- Peak position: Indicates electron binding energy
- Peak area: Proportional to number of electrons in that orbital
Electron Configuration from PES:
Example: Analyzing the PES spectrum of lithium
- Peak at ~0.5 MJ/mol: 2s¹ electron (low binding energy, easily removed)
- Peak at ~6.3 MJ/mol: 1s² electrons (high binding energy, tightly bound)
This confirms Li electron configuration: 1s² 2s¹
Energy Level Interpretations:
Factors Affecting Binding Energy:
- Nuclear Charge: Higher Z → stronger attraction → higher binding energy
- Distance from Nucleus: Farther electrons → lower binding energy
- Shielding: More inner electrons → less effective nuclear charge → lower binding energy
- Penetration: s orbitals penetrate closer to nucleus → higher binding energy than p orbitals
Practice Problem: The PES spectrum of boron shows peaks at approximately 0.8, 1.4, and 19 MJ/mol. Assign these peaks to specific orbitals.
Solution:
- 19 MJ/mol: 1s² (closest to nucleus, highest binding energy)
- 1.4 MJ/mol: 2s² (farther from nucleus, moderate binding energy)
- 0.8 MJ/mol: 2p¹ (same shell as 2s but less penetrating, lowest binding energy)
This confirms B configuration: 1s² 2s² 2p¹
Topic 1.7: Periodic Trends
Learning Objective: Explain the relationship between trends in atomic properties of elements and electronic structure and periodicity.
Four Major Trends:
1. Ionization Energy
Definition: Energy required to remove the most loosely bound electron from a gaseous atom.
Trends:
- Across a period: Increases (stronger nuclear attraction)
- Down a group: Decreases (electrons farther from nucleus)
Exceptions:
- Group 13 vs 12: Slight decrease due to p orbital being higher energy than s
- Group 16 vs 15: Slight decrease due to electron-electron repulsion in paired p orbitals
2. Atomic and Ionic Radii
Definition: Half the distance between nuclei of identical atoms in a bond.
Atomic Radius Trends:
- Across a period: Decreases (stronger nuclear pull)
- Down a group: Increases (additional electron shells)
Ionic Radius Patterns:
- Cations: Smaller than parent atoms (lost electron shell or reduced electron-electron repulsion)
- Anions: Larger than parent atoms (increased electron-electron repulsion)
3. Electron Affinity
Definition: Energy change when an electron is added to a gaseous atom.
Trends:
- Across a period: Generally becomes more negative (stronger nuclear attraction)
- Down a group: Generally becomes less negative (electrons farther from nucleus)
Notable Exception: Group 18 (noble gases) have positive electron affinities due to stable electron configurations.
4. Electronegativity
Definition: Ability of an atom to attract electrons in a chemical bond.
Trends:
- Across a period: Increases (stronger nuclear attraction)
- Down a group: Decreases (electrons farther from nucleus)
- Highest: Fluorine (4.0 on Pauling scale)
- Lowest: Francium (~0.7 on Pauling scale)
Trend Explanations Using Coulomb’s Law:
All periodic trends can be understood through three factors:
- Effective Nuclear Charge (Z_eff):
- Z_eff = Z – S (where Z = nuclear charge, S = shielding constant)
- Higher Z_eff → stronger attraction → smaller radius, higher ionization energy
- Shielding Effect:
- Inner electrons reduce the nuclear charge felt by outer electrons
- More inner electrons → more shielding → weaker nuclear attraction
- Principal Energy Level:
- Higher n → electrons farther from nucleus → weaker nuclear attraction
Prediction Strategies:
Strategy 1: Use position on periodic table
- Same period: trends due to changing nuclear charge
- Same group: trends due to changing distance from nucleus
Strategy 2: Consider electron configurations
- Half-filled and fully-filled subshells provide extra stability
- Electron-electron repulsion affects trends
Practice Problems:
Problem 1: Arrange the following in order of increasing atomic radius: Na, Mg, Al, Si
Solution: Si < Al < Mg < Na (decreasing across period 3)
Problem 2: Which has the higher first ionization energy: N or O?
Solution: N has higher ionization energy due to half-filled p subshell stability (Hund’s rule exception)
Topic 1.8: Valence Electrons and Ionic Compounds
Learning Objective: Explain the relationship between trends in the reactivity of elements and periodicity.
Reactivity Patterns:
Chemical reactivity is primarily determined by valence electron configuration:
- Metals: Tend to lose electrons to achieve noble gas configuration
- Most Reactive: Group 1 (alkali metals)
- Reactivity increases down the group (easier to lose electrons)
- Nonmetals: Tend to gain electrons to achieve noble gas configuration
- Most Reactive: Group 17 (halogens)
- Reactivity decreases down the group (harder to gain electrons)
- Noble Gases: Least reactive due to complete valence shells
Ionic Charge Predictions:
Predictable Charges:
- Group 1: +1 (lose 1 electron)
- Group 2: +2 (lose 2 electrons)
- Group 13: +3 (lose 3 electrons)
- Group 15: -3 (gain 3 electrons)
- Group 16: -2 (gain 2 electrons)
- Group 17: -1 (gain 1 electron)
Transition Metals: Variable charges due to similar energies of ns and (n-1)d orbitals
- Common charges: Fe²⁺/Fe³⁺, Cu⁺/Cu²⁺, Cr²⁺/Cr³⁺
Periodic Family Similarities:
Elements in the same group have similar properties due to identical valence electron configurations:
Group 1 (Alkali Metals):
- All have ns¹ configuration
- Form +1 ions readily
- React vigorously with water: 2M + 2H₂O → 2MOH + H₂
- Reactivity increases: Li < Na < K < Rb < Cs
Group 17 (Halogens):
- All have ns²np⁵ configuration
- Form -1 ions readily
- Exist as diatomic molecules: F₂, Cl₂, Br₂, I₂
- Reactivity decreases: F > Cl > Br > I
Group 18 (Noble Gases):
- All have complete valence shells (ns²np⁶, except He: 1s²)
- Chemically inert under normal conditions
- Higher members can form compounds under extreme conditions
AP-Style Multiple Choice Questions
Question 1: How many moles of oxygen atoms are contained in 2.4 × 10²³ molecules of glucose (C₆H₁₂O₆)?
A) 0.40 mol
B) 1.2 mol
C) 2.4 mol
D) 6.0 mol
Solution:
- Molecules of glucose = 2.4 × 10²³
- Moles of glucose = (2.4 × 10²³) ÷ (6.022 × 10²³) = 0.40 mol
- Each glucose has 6 O atoms
- Moles of O atoms = 0.40 mol × 6 = 2.4 mol
Answer: C
Question 2: Which of the following atoms has the largest atomic radius?
A) Li
B) Na
C) K
D) Rb
Solution: Atomic radius increases down a group due to additional electron shells.
Answer: D
Question 3: The mass spectrum of element X shows two peaks: one at m/z = 79 (50.7% abundance) and another at m/z = 81 (49.3% abundance). What is the average atomic mass of element X?
A) 79.0 amu
B) 79.5 amu
C) 80.0 amu
D) 80.5 amu
Solution:
Average atomic mass = (79 × 0.507) + (81 × 0.493) = 40.05 + 39.93 = 80.0 amu
Answer: C
Question 4: Which electron configuration represents a transition metal in its +2 oxidation state?
A) [Ar] 3d⁶
B) [Ar] 4s² 3d⁶
C) [Kr] 5s²
D) [Ne] 3s²
Solution: Transition metals lose 4s electrons first when forming cations. [Ar] 3d⁶ represents Fe²⁺.
Answer: A
Question 5: Based on Coulomb’s law, which factor most significantly affects the attraction between a nucleus and an electron?
A) The number of neutrons in the nucleus
B) The distance between the nucleus and electron
C) The number of protons in the nucleus
D) The spin of the electron
Solution: F ∝ q₁q₂/r². Nuclear charge (number of protons) directly affects the force.
Answer: C
Free Response Question Practice
FRQ 1: A student analyzes an unknown compound containing only carbon, hydrogen, and oxygen through combustion analysis.
Given data:
- Mass of unknown compound: 1.50 g
- Mass of CO₂ produced: 2.20 g
- Mass of H₂O produced: 0.90 g
(a) Determine the number of moles of carbon and hydrogen in the original sample.
(b) Calculate the mass of oxygen in the original sample.
(c) Determine the empirical formula of the compound.
(d) If the molar mass of the compound is 60.0 g/mol, what is the molecular formula?
Solution:
(a) Moles of carbon and hydrogen:
- Moles of C = moles of CO₂ = 2.20 g ÷ 44.01 g/mol = 0.0500 mol C
- Moles of H = 2 × moles of H₂O = 2 × (0.90 g ÷ 18.02 g/mol) = 0.100 mol H
(b) Mass of oxygen:
- Mass of C = 0.0500 mol × 12.01 g/mol = 0.601 g
- Mass of H = 0.100 mol × 1.008 g/mol = 0.101 g
- Mass of O = 1.50 g – 0.601 g – 0.101 g = 0.798 g
(c) Empirical formula:
- Moles of O = 0.798 g ÷ 16.00 g/mol = 0.0499 mol
- Mole ratio: C:H:O = 0.0500:0.100:0.0499 = 1.00:2.00:1.00
- Empirical formula: CH₂O
(d) Molecular formula:
- Empirical formula mass = 12.01 + 2(1.008) + 16.00 = 30.03 g/mol
- n = 60.0 g/mol ÷ 30.03 g/mol = 2.00
- Molecular formula: C₂H₄O₂
Common Error Analysis
Error 1: Significant Figures
Reporting 0.333333 mol instead of 0.333 mol
Always match significant figures to the least precise measurement
Error 2: Unit Confusion
Using atomic mass (amu) instead of molar mass (g/mol) in calculations
Remember: amu for individual atoms, g/mol for moles of substance
Error 3: Electron Configuration of Ions
Writing Fe³⁺ as [Ar] 4s² 3d³
Remove electrons from highest energy orbitals first: [Ar] 3d⁵
Error 4: Periodic Trend Exceptions
Assuming all trends are perfectly linear
Remember exceptions due to electron configuration (e.g., N vs O ionization energy)
Time Management Tips
Multiple Choice Section (90 minutes, 60 questions):
- Average time per question: 1.5 minutes
- Strategy: Answer easy questions first, mark difficult ones for review
- Calculator use: Not allowed, so practice mental math and estimation
Free Response Section (105 minutes, 7 questions):
- Time allocation: ~15 minutes per question
- Strategy: Read all questions first, start with your strongest topics
- Show work: Partial credit is awarded for correct reasoning
Visual Learning Resources
Interactive Periodic Table Guide
Using the Periodic Table Effectively:
- Electron Configuration Shortcuts:
- s-block: Groups 1-2 (filling s orbitals)
- p-block: Groups 13-18 (filling p orbitals)
- d-block: Groups 3-12 (filling d orbitals)
- f-block: Lanthanides and actinides (filling f orbitals)
- Trend Visualization:
- Atomic radius: Imagine circles getting smaller left-to-right, larger top-to-bottom
- Ionization energy: Imagine energy required increasing left-to-right, decreasing top-to-bottom
- Electronegativity: Fluorine is the “electron magnet” in the top-right corner
Electron Configuration Diagrams
Orbital Filling Order:
1s ↑↓
2s ↑↓ 2p ↑↓ ↑↓ ↑↓
3s ↑↓ 3p ↑↓ ↑↓ ↑↓ 3d ↑↓ ↑↓ ↑↓ ↑↓ ↑↓
4s ↑↓ 4p ↑↓ ↑↓ ↑↓ 4d ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 4f ...Hund’s Rule Visualization:
For nitrogen (7 electrons): 1s² 2s² 2p³
2p: ↑ ↑ ↑ (fill singly first)
NOT: ↑↓ ↑ _ (don't pair until necessary)PES Spectrum Examples
Interpreting PES Data:
Example: Carbon PES Spectrum
- Peak 1 (highest binding energy): 1s² electrons
- Peak 2 (medium binding energy): 2s² electrons
- Peak 3 (lowest binding energy): 2p² electrons
Key Insights:
- Peak height ∝ number of electrons
- Peak position ∝ binding energy
- Multiple peaks in same shell due to different orbital types
Mass Spectrum Interpretations
Reading Mass Spectra:
Example: Chlorine Mass Spectrum
- m/z = 35: ³⁵Cl isotope (75.8% abundance)
- m/z = 37: ³⁷Cl isotope (24.2% abundance)
Molecular Ion Patterns:
- Cl₂: Peaks at m/z = 70, 72, 74 (due to isotope combinations)
- Intensity ratios: Reflect isotope abundance combinations
Trend Visualization Charts
Periodic Trends Summary Table:
| Property | Across Period | Down Group | Explanation |
|---|---|---|---|
| Atomic Radius | Decreases | Increases | Nuclear charge vs electron shells |
| Ionization Energy | Increases | Decreases | Nuclear attraction vs distance |
| Electron Affinity | More negative | Less negative | Nuclear attraction vs distance |
| Electronegativity | Increases | Decreases | Nuclear attraction vs distance |
Study Strategies & Exam Prep
Memory Techniques
Mnemonics for Electron Configuration:
- Orbital Order: “Silly People Don’t Fear Difficult Problems”
- S, P, D, F (orbital types)
- Aufbau Principle: “Always Fill Up Before Going Up”
- Fill lower energy orbitals before higher ones
- Periodic Table Blocks:
- s-block: “Super Simple” (Groups 1-2)
- p-block: “Pretty Popular” (Groups 13-18)
- d-block: “Definitely Different” (Groups 3-12)
- f-block: “Fairly Forgotten” (Lanthanides/Actinides)
Trend Pattern Recognition:
- “DIIE” for Periodic Trends:
- Down groups: atomic radius increases, ionization energy decreases
- In periods: atomic radius decreases, ionization energy increases
- Ionization energy and atomic radius are inversely related
- Electronegativity follows ionization energy pattern
- Noble Gas Stability:
- “Noble gases are noble because they’re complete”
- Use as reference points for electron configurations
Formula Memorization Tips:
- Moles Formula: “Never Forget: n = m/M”
- n (moles), m (mass), M (molar mass)
- Coulomb’s Law: “Force Equals Charges Times Charges Over r-squared”
- F ∝ q₁q₂/r²
- Average Atomic Mass: “Weighted Average = Sum of (Mass × Fraction)”
Practice Schedule
Week 1: Foundational Concepts
Day 1-2: Mole Concept Mastery
- Review Avogadro’s number and molar mass
- Practice dimensional analysis problems
- Focus on unit conversions
Day 3-4: Atomic Structure Basics
- Study electron configuration rules
- Practice writing configurations for elements and ions
- Learn orbital filling order
Day 5-7: Periodic Trends Introduction
- Understand the four major trends
- Practice predicting properties based on position
- Review exceptions and explanations
Week 2: Problem-Solving Focus
Day 8-9: Mass Spectrometry and Isotopes
- Interpret mass spectra
- Calculate average atomic masses
- Understand isotope abundance
Day 10-11: Empirical and Molecular Formulas
- Master combustion analysis problems
- Practice percent composition calculations
- Connect empirical to molecular formulas
Day 12-14: PES and Advanced Concepts
- Interpret photoelectron spectra
- Connect PES data to electron configurations
- Understand binding energy concepts
Week 3: Exam Preparation
Day 15-16: Mixed Practice Problems
- Solve AP-style multiple choice questions
- Time yourself on problem sets
- Identify weak areas for review
Day 17-18: Free Response Practice
- Complete full FRQ problems
- Practice showing work clearly
- Review rubrics and scoring guidelines
Day 19-21: Final Review and Test-Taking Strategies
- Review all formulas and constants
- Practice time management
- Take a full practice exam
AP Exam Specific Tips
Calculator vs Non-Calculator Sections:
Multiple Choice (No Calculator):
- Practice mental math and estimation
- Know common conversion factors by heart
- Use scientific notation effectively
- Round strategically for easier calculations
Free Response (Calculator Allowed):
- Don’t rely too heavily on calculator
- Show mathematical setup clearly
- Use appropriate significant figures
- Check answers for reasonableness
Time Allocation Strategies:
Multiple Choice (90 minutes):
- First pass (45 minutes): Answer questions you’re confident about
- Second pass (30 minutes): Work on moderate difficulty questions
- Final pass (15 minutes): Attempt remaining questions, make educated guesses
Free Response (105 minutes):
- Read all questions (5 minutes): Choose your order
- Easy questions first (40 minutes): Build confidence and momentum
- Moderate questions (45 minutes): Take time for careful analysis
- Difficult questions (15 minutes): Get partial credit where possible
Common Trap Answers:
- Unit Errors: Always check that your answer has the correct units
- Significant Figure Traps: Match precision to given data
- Electron Configuration Mistakes: Remember to remove electrons from highest energy orbitals for cations
- Trend Exceptions: Watch for questions about N vs O, or other known exceptions
- Isotope vs Element: Distinguish between properties of isotopes vs average properties
Advanced Applications
Real-World Chemistry Connections
Nanotechnology and Atomic Structure:
Modern nanotechnology relies heavily on understanding atomic structure:
- Quantum Dots: Semiconductor nanoparticles whose properties depend on size and electron configuration
- Graphene: Single layer of carbon atoms with unique electronic properties due to sp² hybridization
- Carbon Nanotubes: Cylindrical carbon structures with properties determined by atomic arrangement
Medical Applications:
- Radioisotopes in Medicine:
- Technetium-99m: Most widely used medical radioisotope for imaging
- Iodine-131: Used to treat thyroid cancer
- Carbon-14: Dating archaeological specimens
- Magnetic Resonance Imaging (MRI):
- Relies on nuclear magnetic properties of hydrogen atoms
- Different tissues have different hydrogen environments
- Electron configuration affects magnetic properties
Environmental Chemistry:
- Ozone Depletion:
- Understanding halogen reactivity (electron affinity trends)
- Chlorine’s ability to catalyze ozone destruction
- Periodic trends explain why fluorine compounds are less destructive
- Heavy Metal Toxicity:
- Lead and mercury toxicity related to their electron configurations
- Ability to form stable complexes with biological molecules
- Periodic trends predict toxicity patterns
How Unit 1 Connects to Other Units
Unit 2: Molecular and Ionic Compound Structure:
- Electron configurations determine bonding patterns
- Periodic trends predict bond polarities
- Valence electrons determine molecular geometry
Unit 3: Intermolecular Forces and Properties:
- Electronegativity differences determine dipole moments
- Electron configurations affect polarizability
- Atomic size influences van der Waals forces
Unit 4: Chemical Reactions:
- Ionization energy and electron affinity predict reaction spontaneity
- Electron configurations determine oxidation states
- Periodic trends explain reaction patterns
Unit 5: Kinetics:
- Atomic size affects collision frequency
- Electron configuration influences activation energy
- Bond strengths relate to atomic properties
Unit 6: Thermodynamics:
- Ionization energy and electron affinity are thermodynamic quantities
- Lattice energy depends on ionic charges and sizes
- Periodic trends explain enthalpy patterns
College Chemistry Preparation
Advanced Topics You’ll Encounter:
- Quantum Chemistry:
- Schrödinger equation solutions
- Molecular orbital theory
- Spectroscopy and selection rules
- Inorganic Chemistry:
- Coordination compounds and crystal field theory
- Transition metal chemistry
- Solid state chemistry
- Physical Chemistry:
- Statistical thermodynamics
- Kinetic molecular theory
- Advanced spectroscopic methods
Skills That Transfer:
- Mathematical Problem Solving: Dimensional analysis, proportional reasoning
- Model Thinking: Using simplified models to understand complex systems
- Data Interpretation: Analyzing experimental results and spectra
- Trend Recognition: Predicting properties from periodic position
Research Applications
Current Research Areas:
- Materials Science:
- Perovskite Solar Cells: Electron configuration determines photovoltaic properties
- Superconductors: Electronic structure affects superconducting temperature
- Catalysts: Surface electron configuration determines catalytic activity
- Drug Discovery:
- Molecular Recognition: Electron distribution affects binding affinity
- Pharmacokinetics: Molecular properties determine drug behavior
- Toxicology: Electronic structure predicts biological activity
- Environmental Science:
- Atmospheric Chemistry: Radical reactions depend on electron configurations
- Water Treatment: Oxidation-reduction processes rely on electron transfer
- Green Chemistry: Designing environmentally friendly processes
Recommended Resources
Primary Textbooks:
- “Chemistry: The Central Science” by Brown, LeMay, Bursten, Murphy, and Woodward
- Comprehensive coverage with excellent problem sets
- Strong emphasis on conceptual understanding
- Available in international editions
- “Chemistry” by Zumdahl and Zumdahl
- Clear explanations with step-by-step problem solving
- Good balance of theory and applications
- Extensive online resources
- “Chemistry: A Molecular Approach” by Tro
- Modern approach with emphasis on molecular-level understanding
- Excellent visual aids and animations
- Strong connection between concepts and applications
AP-Specific Resources:
- “5 Steps to a 5: AP Chemistry” by Richard H. Langley
- Focused AP exam preparation
- Practice tests with detailed explanations
- Study plans for different preparation timelines
- “Cracking the AP Chemistry Exam” by The Princeton Review
- Test-taking strategies and tips
- Content review with practice problems
- Full-length practice exams
Online Simulators
Interactive Learning Tools:
- PhET Simulations (University of Colorado Boulder):
- Build an Atom: Interactive atom construction
- Isotopes and Atomic Mass: Mass spectrometry simulation
- Photoelectron Spectroscopy: Virtual PES experiments
- Free and available in multiple languages
- ChemSketch (ACD/Labs):
- Draw molecular structures and electron configurations
- Calculate molecular properties
- Free for academic use
- Avogadro (Open Source):
- 3D molecular visualization
- Build and manipulate atomic structures
- Calculate electronic properties
Virtual Laboratory Experiences:
- ChemCollective (Carnegie Mellon University):
- Virtual lab experiments
- Stoichiometry and analytical chemistry simulations
- Real-time data collection and analysis
- Late Nite Labs:
- Professional virtual chemistry labs
- Atomic structure and periodic trends experiments
- Detailed lab reports and data analysis
Practice Exam Resources
Official College Board Materials:
- AP Chemistry Course and Exam Description:
- Official learning objectives and essential knowledge
- Sample questions with scoring guidelines
- Updated annually with any curriculum changes
- AP Classroom:
- Teacher and student resources
- Progress checks and practice questions
- Personal progress tracking
- Past AP Exams:
- Released free response questions (1999-present)
- Official scoring guidelines and sample responses
- Multiple choice questions from select years
International Perspectives:
- Royal Society of Chemistry (UK):
- Professional chemistry content
- Current research applications
- International chemistry standards
- American Chemical Society:
- Educational resources and videos
- Career guidance in chemistry
- Current events in chemistry
FAQ: Top 10 Student Questions
Q1: How do I memorize all the electron configurations?
A: Don’t memorize them all! Instead, learn the pattern:
- Use the periodic table as your guide (s, p, d, f blocks)
- Practice the aufbau principle with the diagonal rule
- Focus on common ions and transition metals
- Use noble gas notation for efficiency
- Remember that practice makes permanent, not perfect
Q2: Why does the electron configuration of chromium end in 4s¹3d⁵ instead of 4s²3d⁴?
A: This is due to electron stability:
- Half-filled d subshells (d⁵) are particularly stable
- The energy difference between 4s and 3d orbitals is small
- Electron-electron repulsion is minimized in the half-filled configuration
- Similar exceptions occur with copper (4s¹3d¹⁰) for the same stability reasons
Q3: How do I know which periodic trend to apply in a given problem?
A: Look for key phrases in the question:
- “Size” or “radius” → atomic/ionic radius trends
- “Energy to remove electron” → ionization energy trends
- “Attraction for electrons” → electronegativity trends
- “Energy released when gaining electron” → electron affinity trends
- Always consider both horizontal (across periods) and vertical (down groups) trends
Q4: What’s the difference between empirical and molecular formulas?
A:
- Empirical formula: Simplest whole-number ratio of atoms (CH₂O)
- Molecular formula: Actual number of atoms in a molecule (C₆H₁₂O₆)
- Relationship: Molecular = Empirical × n (where n is a whole number)
- From combustion analysis: You always get empirical first, then need molar mass for molecular
Q5: How do I interpret photoelectron spectroscopy (PES) data?
A: Follow this systematic approach:
- x-axis: Binding energy (higher = more tightly bound electrons)
- y-axis: Number of electrons (peak area/height)
- Peak positions: Match to known orbital energies (1s > 2s > 2p > 3s…)
- Peak areas: Should match electron count in each orbital
- Multiple peaks in same shell: Different orbital types (s vs p)
Q6: Why do some elements not follow the expected periodic trends?
A: Exceptions occur due to:
- Electron configuration effects: Half-filled and filled subshells are extra stable
- Shielding variations: d electrons shield differently than s and p electrons
- Orbital penetration: s orbitals penetrate closer to nucleus than p orbitals
- Electron-electron repulsion: Pairing electrons in orbitals costs energy
- These exceptions actually prove the underlying theory is correct!
Q7: How do I approach combustion analysis problems systematically?
A: Use this step-by-step method:
- Find moles of C and H from CO₂ and H₂O products
- Calculate masses of C and H in original sample
- Find mass of O by difference (if compound contains O)
- Convert all masses to moles
- Find simplest whole-number ratio
- If given molar mass, find molecular formula
Q8: What’s the best way to study for the AP Chemistry exam?
A: Follow the 3-2-1 approach:
- 3 weeks before: Content review and concept mastery
- 2 weeks before: Mixed practice problems and weak area focus
- 1 week before: Full practice exams and test-taking strategies
- Daily: Review formulas, do practice problems, check understanding
- Focus on understanding, not memorization
Q9: How do I manage my time effectively on the AP exam?
A:
Multiple Choice (90 min, 60 questions):
- Aim for 1.5 minutes per question
- Answer easy questions first (builds confidence)
- Mark difficult ones for later review
- No penalty for guessing, so answer everything
Free Response (105 min, 7 questions):
- ~15 minutes per question
- Read all questions first, start with strongest topics
- Show all work clearly for partial credit
- If stuck, move on and return later
Q10: How does Unit 1 connect to the rest of AP Chemistry?
A: Unit 1 is the foundation for everything:
- Bonding (Unit 2): Electron configurations determine bonding patterns
- Intermolecular Forces (Unit 3): Atomic properties affect molecular interactions
- Reactions (Unit 4): Periodic trends predict reaction behavior
- Kinetics (Unit 5): Atomic size and electron configuration affect reaction rates
- Thermodynamics (Unit 6): Atomic properties determine energy changes
- Equilibrium (Unit 7): Electronic structure affects equilibrium positions
Clarifications on Tricky Concepts
Concept: Effective Nuclear Charge
Student Confusion: “How do I calculate effective nuclear charge?”
Clarification:
- Simple approximation: Z_eff = Z – S (nuclear charge minus shielding)
- Slater’s rules provide more accurate shielding constants
- For AP Chemistry: Focus on trends, not exact calculations
- Key insight: Inner electrons shield outer electrons from nuclear attraction
Concept: Ionic vs Atomic Radius
Student Confusion: “Why are some ionic radii larger than atomic radii?”
Clarification:
- Cations: Smaller than parent atoms (lost electrons, less repulsion)
- Anions: Larger than parent atoms (gained electrons, more repulsion)
- Isoelectronic series: Same number of electrons, different nuclear charges
- Example: O²⁻ > F⁻ > Na⁺ > Mg²⁺ (all have 10 electrons)
Concept: First vs Second Ionization Energy
Student Confusion: “Why is the second ionization energy always higher?”
Clarification:
- Removing from a positive ion: Harder due to increased attraction
- Fewer electrons: Less electron-electron repulsion
- Same nuclear charge: Pulling on fewer electrons more strongly
- Big jumps: Occur when removing from a new electron shell
Exam Format Questions
Q: What formulas and constants are provided on the AP exam?
A: The AP Chemistry equation sheet includes:
- Constants: Avogadro’s number, gas constant, Planck’s constant
- Equations: For thermodynamics, kinetics, equilibrium, etc.
- NOT provided: Specific heat values, Ka/Kb values, standard potentials
- Unit 1 specific: Avogadro’s number is provided, mole relationships are not
Q: Can I use a calculator on the multiple choice section?
A: No, calculators are not allowed on the multiple choice section:
- Practice mental math and estimation
- Know common conversion factors
- Use scientific notation effectively
- Round numbers to make calculations easier
Q: How much partial credit is awarded on free response questions?
A: Substantial partial credit is available:
- Show your work: Even incorrect final answers can earn points
- Correct reasoning: Logical approach earns points even with calculation errors
- Units and significant figures: Usually worth points
- Clear communication: Well-explained answers earn more points
Study Timeline Concerns
Q: I only have 2 weeks to study for the AP exam. What should I focus on?
A: Prioritize high-impact topics:
- Week 1: Focus on major concepts (moles, electron configuration, periodic trends)
- Week 2: Practice problems and exam strategies
- Skip: Detailed history and derivations
- Emphasize: Problem-solving techniques and common question types
Q: How much time should I spend on Unit 1 compared to other units?
A: Unit 1 timing recommendations:
- Total study time: 15-20% of your AP Chemistry preparation
- Rationale: 7-9% of exam, but foundation for everything else
- Focus areas: Concepts that appear in later units (electron configuration, trends)
- Less emphasis: Purely memorization-based content
Q: Should I study topics in the order they appear in the curriculum?
A: Generally yes, but with flexibility:
- Unit 1 first: Essential foundation for all other topics
- Build connections: See how Unit 1 concepts apply in later units
- Review regularly: Return to Unit 1 concepts when studying later units
- Spiral approach: Deepen understanding with each review
Conclusion: Key Takeaways Summary
Congratulations! You’ve completed a comprehensive journey through AP Chemistry Unit 1: Atomic Structure and Properties. Let’s consolidate the most important insights that will serve you throughout your chemistry studies and beyond.
Essential Concepts Mastered:
- The Mole Concept: You now understand chemistry’s most powerful tool for connecting the microscopic and macroscopic worlds. Remember that n = m/M is more than just a formula-it’s the bridge between atoms and grams.
- Electron Configuration: The aufbau principle, Pauli exclusion principle, and Hund’s rule aren’t just memorization tasks. They explain why elements behave the way they do and predict chemical properties.
- Periodic Trends: The four major trends (atomic radius, ionization energy, electron affinity, and electronegativity) all stem from Coulomb’s law and the balance between nuclear attraction and electron-electron repulsion.
- Experimental Techniques: Mass spectrometry and photoelectron spectroscopy provide experimental evidence for atomic structure theory. These aren’t just abstract concepts-they’re tools used daily in research labs worldwide.
Problem-Solving Skills Developed:
- Dimensional Analysis: The systematic approach to unit conversion and stoichiometry
- Data Interpretation: Reading and analyzing mass spectra and PES data
- Trend Prediction: Using periodic position to predict atomic properties
- Mathematical Modeling: Applying Coulomb’s law to explain atomic behavior
Real-World Connections Established:
You’ve seen how Unit 1 concepts apply to:
- Nanotechnology: Quantum dots and graphene applications
- Medicine: Radioisotopes and MRI technology
- Environmental Science: Ozone depletion and pollution chemistry
- Materials Science: Superconductors and catalysts
Study Strategies Internalized:
- Active Learning: Using mnemonics, visual aids, and practice problems
- Spaced Repetition: Regular review to build long-term retention
- Error Analysis: Learning from mistakes to avoid common pitfalls
- Time Management: Efficient exam strategies for maximum performance
Direct Connections:
- Electron Configuration → Bonding Patterns:
- Valence electrons determine how atoms bond
- Electron configurations predict ionic charges
- Half-filled and filled subshells affect bonding preferences
- Periodic Trends → Bond Properties:
- Electronegativity differences determine bond polarity
- Atomic size affects bond length and strength
- Ionization energy predicts metallic vs nonmetallic behavior
- Coulomb’s Law → Lattice Energy:
- Same F ∝ q₁q₂/r² relationship applies to ionic compounds
- Charge and size determine ionic compound stability
- Explains trends in melting points and solubility
New Concepts to Anticipate:
- Lewis Structures: Using valence electrons to predict molecular structure
- VSEPR Theory: How electron pairs determine molecular geometry
- Hybridization: Mixing atomic orbitals to explain bonding
- Intermolecular Forces: How atomic properties affect molecular interactions
Preparation Tips for Unit 2:
- Keep your periodic table handy-you’ll use it constantly
- Review electron configuration of ions regularly
- Practice predicting charges based on electron configuration
- Remember that atoms “want” to achieve noble gas configurations
Final Motivation
As you move forward in your AP Chemistry journey, remember that Unit 1 isn’t just the first chapter-it’s the foundation upon which all of chemistry is built. Every concept you’ll encounter, from complex organic reactions to industrial processes, ultimately comes down to how electrons behave around atomic nuclei.
Your Chemistry Future:
Whether you’re planning a career in:
- Medicine: Understanding drug interactions at the molecular level
- Engineering: Designing new materials with specific properties
- Environmental Science: Solving pollution and sustainability challenges
- Research: Pushing the boundaries of scientific knowledge
- Education: Inspiring the next generation of scientists
The principles you’ve mastered in Unit 1 will be your constant companions.
Success Mindset:
Remember these key principles as you continue:
- Chemistry is logical: Every trend and exception has an underlying explanation
- Practice builds intuition: The more problems you solve, the more patterns you’ll recognize
- Mistakes are learning opportunities: Each error teaches you something valuable
- Connections matter: Always look for relationships between concepts
- Curiosity drives understanding: Ask “why” and “how” questions constantly
Remember: You’re not just studying for an exam-you’re developing the scientific literacy to understand and shape the world around you. The atomic structure principles you’ve mastered are the same ones used by researchers developing new medications, engineers creating stronger materials, and environmental scientists protecting our planet.
Your journey in chemistry has just begun, and you’re already equipped with powerful tools for understanding the molecular world. Keep that curiosity alive, stay persistent in your studies, and remember that every expert was once a beginner who refused to give up.
Good luck with your AP Chemistry exam, and welcome to the fascinating world of molecular science!
Also Read –

3 thoughts on “AP Chemistry Unit 1: Complete Guide to Atomic Structure & Properties – 2025”