When Our Waters and Lands Cry for Help
Picture this: You’re standing on the shores of Lake Erie in the 1960s, and instead of crystal-clear water, you see a thick, green, toxic soup stretching to the horizon. Fish are floating belly-up, and the smell is overwhelming. This wasn’t science fiction-this was reality. Lake Erie was so polluted it was declared “dead,” and in 1969, Cleveland’s Cuyahoga River actually caught fire due to oil and chemical contamination.

Fast forward to today, and while Lake Erie has made a remarkable recovery, pollution remains one of our most pressing environmental challenges. Every day, we’re making choices that either contribute to or help solve pollution problems in our aquatic and terrestrial ecosystems. From the plastic water bottle you might be drinking from right now to the fertilizer used on the lawn outside your school, these seemingly small actions ripple through complex environmental systems.
Welcome to AP Environmental Science Unit 8: Aquatic and Terrestrial Pollution-where we’ll explore how human activities impact our planet’s water bodies and land ecosystems, and more importantly, what we can do about it. This unit isn’t just about memorizing types of pollutants; it’s about understanding the intricate web of cause and effect that shapes our environment and preparing you to be an informed environmental steward.
Did You Know? The Great Pacific Garbage Patch, a massive collection of marine debris, is estimated to be twice the size of Texas and contains at least 80,000 metric tons of plastic!
In this comprehensive guide, you’ll master the fundamental concepts of pollution science, explore real-world case studies that bring textbook theories to life, and develop the critical thinking skills needed to excel on the AP Environmental Science exam. We’ll journey from microscopic pollutants to global-scale environmental disasters, connecting the dots between individual actions and planetary health.
Whether you’re aiming for that perfect 5 on the APES exam or simply want to understand how pollution affects the world around you, this guide will be your roadmap to success. Let’s dive in and discover how pollution science can help us protect the only planet we call home.
Fundamental Concepts: The Science Behind Pollution
Understanding Pollution: More Than Just “Dirty” Water and Soil
Before we dive into the nitty-gritty details, let’s establish what pollution actually means in environmental science terms. Pollution is the introduction of harmful substances or energy into the environment at rates faster than natural systems can break them down, dilute them, or recycle them into harmless forms. Think of it like your bedroom-if you throw clothes on the floor faster than you can clean them up, eventually you’ll have a pollution problem!
The key here is the concept of environmental resistance-every ecosystem has a natural capacity to absorb and process waste, but when we exceed that capacity, pollution occurs. This is why understanding carrying capacity and natural cycles is so crucial in environmental science.
Types of Pollutants: The Usual Suspects
Environmental scientists classify pollutants in several ways, and understanding these classifications is essential for the AP exam:
By Degradability:
- Biodegradable pollutants break down naturally through biological processes. Examples include human sewage, food waste, and paper products. These pollutants cause problems when they’re released faster than nature can break them down.
- Non-biodegradable pollutants persist in the environment indefinitely. Plastics, heavy metals like mercury and lead, and synthetic chemicals fall into this category. These are particularly concerning because they accumulate over time.
By Chemical Composition:
- Organic pollutants contain carbon and often come from living sources. Examples include oil, pesticides, and sewage.
- Inorganic pollutants lack carbon in their molecular structure. Heavy metals, acids, and salts are common inorganic pollutants.
By Source:
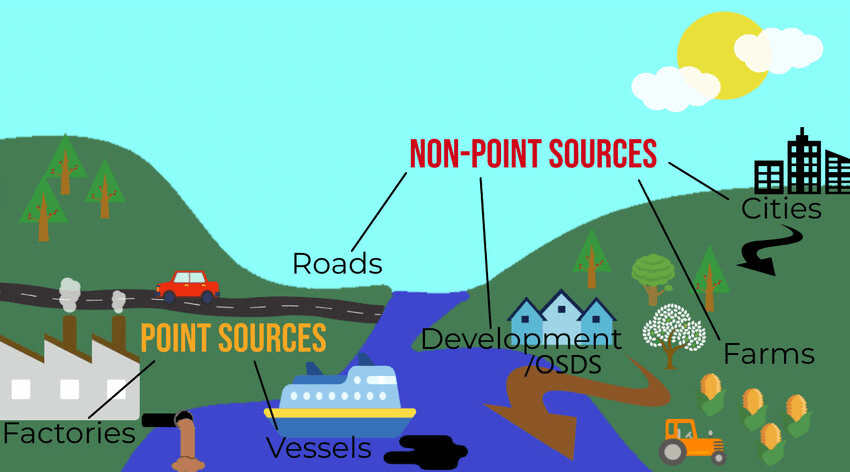
- Point source pollution comes from a single, identifiable location like a factory discharge pipe or an oil spill. These are easier to regulate and control.
- Nonpoint source pollution comes from diffuse, widespread sources like agricultural runoff or urban stormwater. These are much harder to control and represent about 65% of water pollution in the United States.
Aquatic Pollution: When Water Systems Go Wrong
Water pollution affects every type of aquatic ecosystem, from the smallest stream to the vast oceans. Let’s explore the major categories and their impacts:
Nutrient Pollution and Eutrophication
One of the most widespread water pollution problems is eutrophication-the process by which water bodies become overly enriched with nutrients, leading to excessive plant and algae growth. Here’s how it works:
- Nutrient Input: Excess nitrogen and phosphorus enter water bodies from sources like agricultural fertilizer, sewage, and detergents.
- Algae Boom: These nutrients act like fertilizer for aquatic plants and algae, causing explosive growth called algae blooms.
- Oxygen Depletion: When the algae die, bacteria decompose them, consuming oxygen in the process. This creates hypoxic conditions (low oxygen) or even anoxic conditions (no oxygen).
- Dead Zones: Fish and other aquatic life can’t survive without oxygen, creating areas called dead zones where nothing can live.
The Gulf of Mexico dead zone, caused primarily by nutrient runoff from Midwest agriculture, covers an area roughly the size of Connecticut and varies seasonally based on rainfall and farming practices.
Study Tip: Remember the eutrophication process with the acronym “NABO”-Nutrients, Algae, Bacteria, Oxygen depletion.
Toxic Chemical Pollution
Chemical pollutants in water systems pose serious threats to both aquatic life and human health. Key categories include:
Heavy Metals: Mercury, lead, cadmium, and chromium can accumulate in food chains through bioaccumulation and biomagnification. Mercury from coal-fired power plants, for example, settles in water bodies where it’s converted to methylmercury by bacteria. This toxic form then concentrates as it moves up the food chain, reaching dangerous levels in top predators like large fish and marine mammals.
Persistent Organic Pollutants (POPs): These synthetic chemicals resist environmental breakdown and can travel vast distances through air and water currents. DDT, PCBs, and dioxins are classic examples. Even though DDT was banned in the United States in 1972, it’s still found in Antarctic penguins due to global atmospheric transport!
Endocrine Disruptors: These chemicals interfere with hormone systems in wildlife and humans. Common sources include plastics (BPA), pesticides (atrazine), and pharmaceuticals that pass through wastewater treatment systems.
Thermal Pollution
Often overlooked, thermal pollution occurs when human activities significantly alter the temperature of natural water bodies. Power plants and industrial facilities use water for cooling, then discharge it at elevated temperatures. Even small temperature increases can:
- Reduce dissolved oxygen levels (warm water holds less oxygen)
- Disrupt fish spawning and migration patterns
- Alter species composition in aquatic communities
- Increase susceptibility to disease and parasites
Terrestrial Pollution: When Land Systems Suffer
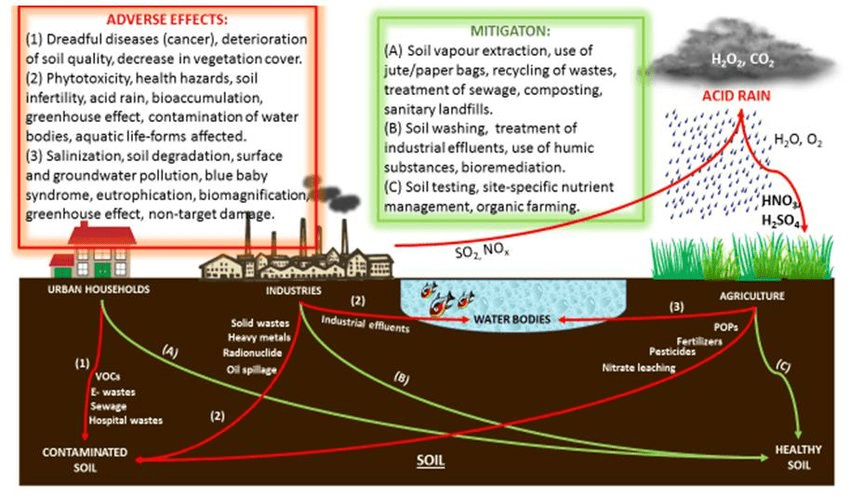
Land pollution affects soil quality, vegetation, and the countless organisms that depend on healthy terrestrial ecosystems. Unlike water pollution, which can be diluted and may flow away, soil pollution tends to persist and accumulate over time.
Soil Contamination Sources and Impacts
Agricultural Pollution: Modern agriculture is a major source of terrestrial pollution through:
- Pesticide residues that can persist in soil for years
- Excess fertilizers that alter soil chemistry and can run off into water systems
- Soil compaction from heavy machinery that reduces soil’s ability to absorb water and support plant growth
- Monoculture practices that reduce biodiversity and soil health
Industrial Contamination: Factories, mining operations, and waste disposal sites can introduce heavy metals, solvents, and other toxic chemicals into soil. Superfund sites-locations contaminated with hazardous substances-require extensive cleanup efforts that can take decades and cost millions of dollars.
Urban Pollution: Cities create unique pollution challenges including:
- Salt contamination from road de-icing that affects roadside vegetation
- Heavy metal deposition from vehicle emissions
- Soil sealing where impermeable surfaces prevent natural water infiltration
- Heat island effects that alter local temperature and precipitation patterns
Solid Waste and Landfills
The average American generates about 4.5 pounds of municipal solid waste per day, and managing this waste stream presents enormous environmental challenges:
Landfill Design and Function: Modern sanitary landfills use engineered systems including:
- Clay liners and synthetic membranes to prevent leachate from reaching groundwater
- Leachate collection systems to capture and treat contaminated water
- Gas collection systems to capture methane produced by decomposing organic matter
- Daily cover to reduce odors, control pests, and minimize air pollution
Waste Stream Composition: Understanding what’s in our waste helps us develop better management strategies:
- Organic materials (food scraps, yard trimmings): ~30%
- Paper and cardboard: ~25%
- Plastics: ~13%
- Metals: ~9%
- Glass: ~4%
- Wood: ~6%
- Textiles: ~5%
- Other materials: ~8%
Chemical Fate and Transport: Following the Pollution Trail
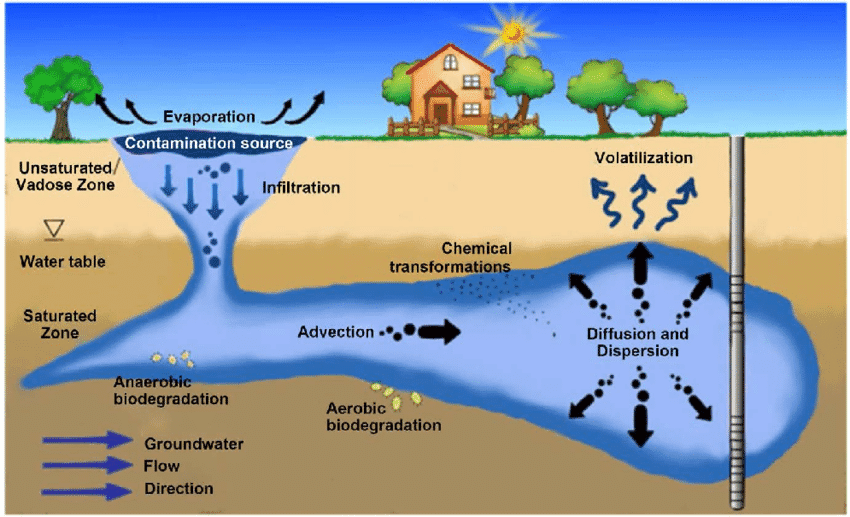
Understanding how pollutants move through environmental systems is crucial for predicting impacts and developing control strategies. Environmental scientists study several key processes:
Volatilization: Some chemicals evaporate from water or soil surfaces and enter the atmosphere. This is how local pollution can become regional or even global problems.
Sorption: Pollutants can bind to soil particles or sediments, which may immobilize them but also create long-term contamination issues.
Biodegradation: Microorganisms can break down some pollutants, but the rate depends on factors like temperature, pH, oxygen availability, and the specific chemical structure.
Bioaccumulation and Biomagnification: These processes concentrate pollutants in living tissues:
- Bioaccumulation occurs when an organism absorbs pollutants faster than it can eliminate them
- Biomagnification occurs when pollutant concentrations increase at successive levels of the food chain
Dose-Response Relationships: The Foundation of Toxicology
In environmental science, we need to understand not just whether a substance is toxic, but how toxic it is at different concentrations. This relationship is described by dose-response curves, which plot the magnitude of response against the dose or concentration of a substance.
Key concepts include:
- Threshold dose: The minimum dose that produces a detectable response
- LD₅₀: The dose that kills 50% of test organisms (Lethal Dose 50)
- ED₅₀: The dose that produces a specific effect in 50% of test organisms (Effective Dose 50)
- NOAEL: No Observed Adverse Effect Level-the highest dose that produces no adverse effects
Did You Know? Paracelsus, a 16th-century physician, stated “The dose makes the poison”-meaning that any substance can be toxic at high enough concentrations, while even known poisons may be harmless at very low doses.
Real-World Applications: Pollution in Action
Case Study 1: The Flint Water Crisis-When Infrastructure Fails
The Flint water crisis exemplifies how environmental injustice, aging infrastructure, and cost-cutting decisions can create devastating pollution problems. In 2014, the city of Flint, Michigan, switched its water supply from Lake Huron to the Flint River as a cost-saving measure. However, the river water was more corrosive than the previous supply and wasn’t properly treated with anti-corrosive agents.
The Chemistry Behind the Crisis:
The Flint River water had different chemical properties than Lake Huron water:
- Higher chloride concentrations
- Different pH levels
- More organic matter
Without proper corrosion control treatment, this water began dissolving the protective mineral scale inside old lead pipes and lead service lines. Lead leached into the drinking water, reaching levels far above EPA safety standards.
Health Impacts:
- Elevated blood lead levels in children, particularly those under 5 years old
- Increased risk of neurological development problems
- Pregnant women and infants faced the highest risks
- Long-term cognitive and behavioral effects in affected children
Environmental Justice Dimensions:
Flint’s population is majority African American and has high poverty rates. The crisis highlighted how marginalized communities often bear disproportionate environmental burdens and may have less political power to demand quick solutions.
Lessons Learned:
- Infrastructure investment is environmental protection
- Water treatment must account for source water chemistry
- Environmental monitoring and transparency are essential
- Community voices matter in environmental decision-making
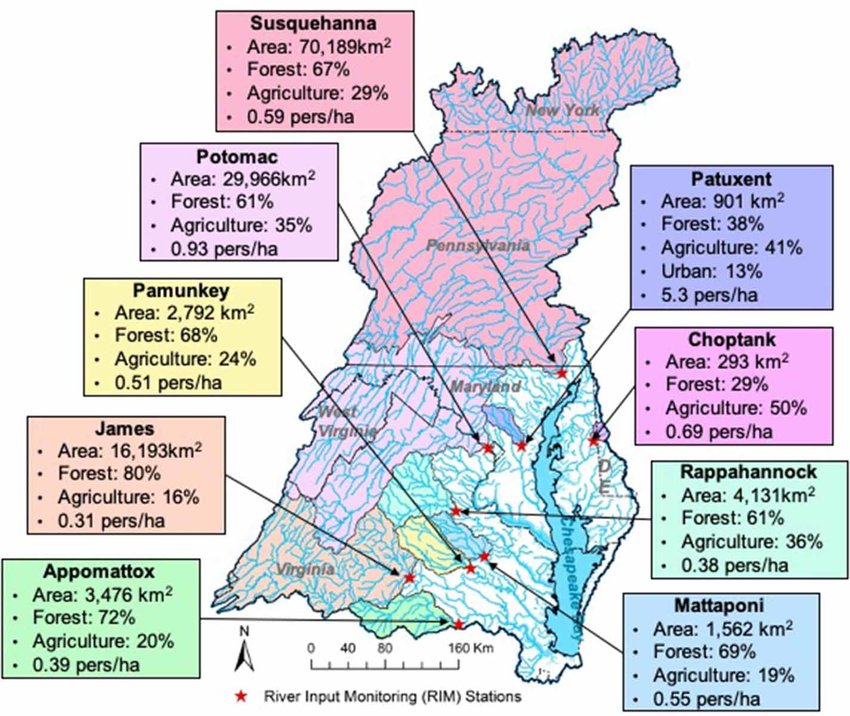
Case Study 2: Agricultural Runoff in the Chesapeake Bay
The Chesapeake Bay, the largest estuary in the United States, has struggled with pollution from agricultural runoff for decades. The bay’s watershed spans six states and includes 64,000 square miles of land, much of it devoted to agriculture.
The Problem:
Excess nitrogen and phosphorus from fertilizers, animal waste, and other sources flow into the bay, causing:
- Massive algae blooms that block sunlight
- Oxygen depletion that creates dead zones
- Loss of underwater grass beds that provide habitat
- Decline in fish, crab, and oyster populations
The Numbers:
- The bay receives approximately 300 million pounds of nitrogen annually
- About 40% comes from agriculture
- The dead zone can cover up to 40% of the bay’s main stem during summer months
Solution Strategies:
The Chesapeake Bay Program represents one of the most comprehensive watershed restoration efforts in the world:
Agricultural Best Management Practices:
- Cover crops planted after harvest to absorb excess nutrients
- Riparian buffers-strips of vegetation along waterways that filter runoff
- Precision agriculture that applies fertilizers more efficiently
- Integrated pest management to reduce pesticide use
Urban Solutions:
- Green infrastructure like rain gardens and permeable pavement
- Stormwater management systems that capture and treat runoff
- Wastewater treatment upgrades to remove more nutrients
Results and Ongoing Challenges:
While significant progress has been made, the bay still faces challenges:
- Climate change is increasing storm intensity and frequency
- Population growth continues in the watershed
- Agricultural practices are slow to change
- Legacy nutrients stored in sediments continue to be released
Case Study 3: Electronic Waste and the Global Pollution Trade
Every year, the world generates over 50 million tons of electronic waste (e-waste), making it the fastest-growing waste stream globally. The way we handle this waste creates pollution problems that span continents.
The E-Waste Journey:
- Generation: Consumers in wealthy countries discard electronics
- Collection: Some e-waste goes to certified recyclers, but much is exported
- Export: E-waste often ends up in developing countries under the guise of “reuse”
- Informal Processing: Workers in places like Agbogbloshie, Ghana, and Guiyu, China, manually dismantle electronics to recover valuable metals
Environmental and Health Impacts:
- Toxic exposure: Workers burn cables to recover copper, releasing dioxins and furans
- Soil contamination: Heavy metals like lead, mercury, and cadmium accumulate in soil
- Water pollution: Acid baths used to extract precious metals contaminate local water supplies
- Air pollution: Open burning releases toxic compounds into the atmosphere
The Chemistry of E-Waste:
Electronic devices contain numerous hazardous substances:
- Lead in older cathode ray tube monitors and solder
- Mercury in LCD backlights and switches
- Cadmium in rechargeable batteries and circuit boards
- Brominated flame retardants in plastic components
- Precious metals like gold, silver, and platinum in circuit boards
Solutions and Challenges:
- Extended Producer Responsibility programs make manufacturers responsible for product end-of-life
- Right to Repair legislation could extend device lifespans
- Urban mining technologies can recover materials more safely
- International cooperation is needed to prevent waste dumping
Case Study 4: Microplastics-The Invisible Pollution Crisis
Microplastics-plastic particles smaller than 5 millimeters-represent a new frontier in pollution science. These tiny particles are now found everywhere from the deepest ocean trenches to the highest mountain peaks.
Sources of Microplastics:
- Primary microplastics: Released directly as small particles (microbeads in cosmetics, synthetic clothing fibers)
- Secondary microplastics: Result from breakdown of larger plastic items due to UV radiation, wave action, and other environmental factors
Environmental Distribution:
- Marine environments: Concentrated in ocean gyres, incorporated into marine food webs
- Freshwater systems: Found in rivers, lakes, and even bottled water
- Terrestrial environments: Present in agricultural soils, often from sewage sludge application
- Atmospheric transport: Microplastics can travel thousands of miles through air currents
Ecological Impacts:
Research is still emerging, but potential impacts include:
- Physical harm: Particles can cause internal damage to organisms
- Chemical effects: Plastics can carry toxic chemicals and release them inside organisms
- Ecosystem disruption: Changes in food web dynamics and energy transfer
Human Health Concerns:
- Microplastics have been found in human blood, lungs, and placental tissue
- Potential for carrying pathogens and toxic chemicals into the human body
- Long-term health effects are still being studied
Environmental Connections: The Web of Pollution
Pollution and Climate Change: A Two-Way Street
The relationship between pollution and climate change is complex and interconnected. Understanding these connections is crucial for developing effective environmental policies.
How Pollution Contributes to Climate Change:
- Greenhouse gas emissions from fossil fuel combustion
- Black carbon (soot) that absorbs solar radiation and accelerates ice melting
- Methane emissions from landfills and agricultural operations
- Deforestation that reduces carbon sequestration capacity
How Climate Change Affects Pollution:
- Increased temperatures can worsen air quality by accelerating chemical reactions that form ground-level ozone
- Changing precipitation patterns affect the transport and concentration of pollutants
- Sea level rise can mobilize coastal contamination sites
- Extreme weather events can overwhelm pollution control infrastructure
Pollution and Biodiversity Loss: Interconnected Crises
Pollution is one of the five main drivers of biodiversity loss, alongside habitat destruction, climate change, overexploitation, and invasive species.
Direct Impacts on Species:
- Toxicity: Pollutants can kill organisms directly or reduce their reproductive success
- Habitat degradation: Pollution makes environments unsuitable for native species
- Food web disruption: Pollutants can eliminate key species, disrupting entire ecosystems
Case Example-Bee Colony Collapse:
Multiple pollutants contribute to declining bee populations:
- Neonicotinoid pesticides affect bee navigation and immune systems
- Fungicides interact synergistically with other chemicals
- Air pollution interferes with flower scents that bees use for navigation
- Habitat fragmentation forces bees to travel through polluted landscapes
The Pollution-Poverty Connection
Environmental pollution and poverty are intimately linked through what environmental scientists call the “pollution-poverty nexus.”
How Pollution Perpetuates Poverty:
- Health costs from pollution-related diseases drain family resources
- Reduced agricultural productivity from soil and water pollution
- Limited economic opportunities in heavily polluted areas
- Educational impacts when children suffer from pollution-related health problems
Why Poor Communities Face More Pollution:
- Environmental racism: Hazardous facilities are disproportionately located in minority and low-income communities
- Less political power to oppose polluting facilities
- Economic necessity may force acceptance of polluting industries for jobs
- Limited resources for pollution control and cleanup
Transboundary Pollution: When Problems Cross Borders
Pollution doesn’t respect political boundaries, creating challenges for environmental governance.
Atmospheric Transport:
- Acid rain caused by sulfur dioxide emissions can affect areas hundreds of miles from the source
- Mercury emissions from coal plants can contaminate fish in remote lakes
- Persistent organic pollutants can travel to polar regions through atmospheric currents
Water-Based Transport:
- River pollution affects downstream countries
- Ocean currents carry pollutants across basins
- Groundwater contamination can cross national borders
International Cooperation Examples:
- Montreal Protocol: Successfully reduced ozone-depleting substances
- Stockholm Convention: Addresses persistent organic pollutants globally
- MARPOL Convention: Regulates ship-based marine pollution
- Great Lakes Water Quality Agreement: US-Canada cooperation on shared water resources
Current Research and Trends: The Cutting Edge of Pollution Science
Emerging Contaminants: The New Challenges
Environmental scientists are increasingly concerned about emerging contaminants-pollutants that are newly recognized as environmental threats or have recently been detected in the environment.
Pharmaceuticals and Personal Care Products (PPCPs):
These compounds pass through wastewater treatment systems and accumulate in aquatic environments:
- Antibiotics can promote antibiotic resistance in environmental bacteria
- Hormones from birth control pills affect fish reproduction
- Antidepressants alter behavior in aquatic organisms
- Nanoparticles from sunscreens and cosmetics have unknown long-term effects
PFAS-The “Forever Chemicals”:
Per- and polyfluoroalkyl substances (PFAS) are synthetic chemicals used in:
- Non-stick cookware coatings
- Water-resistant clothing and equipment
- Food packaging materials
- Firefighting foams
PFAS are concerning because they:
- Don’t break down naturally (hence “forever chemicals”)
- Accumulate in organisms and the environment
- Are linked to various health problems
- Are found in drinking water supplies worldwide
Antimicrobial Resistance:
The overuse of antibiotics in medicine and agriculture has led to the emergence of antibiotic-resistant bacteria in environmental systems. Wastewater treatment plants can serve as “mixing chambers” where resistance genes spread between bacterial species.
Advanced Treatment Technologies
Researchers are developing innovative approaches to pollution control and remediation:
Bioremediation:
Using living organisms to clean up contamination:
- Phytoremediation: Plants that absorb, concentrate, or break down pollutants
- Mycoremediation: Fungi that can break down complex organic pollutants
- Enhanced biodegradation: Adding nutrients or microorganisms to accelerate natural breakdown processes
Nanotechnology Applications:
- Nanoscale zero-valent iron for groundwater treatment
- Photocatalytic nanoparticles that break down organic pollutants using sunlight
- Nanofilters for water treatment systems
Advanced Oxidation Processes:
These technologies use powerful oxidizing agents to break down resistant pollutants:
- Ozonation combined with UV light or hydrogen peroxide
- Fenton processes using iron catalysts
- Electrochemical oxidation systems
Sensor Networks and Environmental Monitoring
Modern pollution monitoring is being revolutionized by new technologies:
Real-Time Monitoring:
- Wireless sensor networks provide continuous data on air and water quality
- Satellite monitoring can track pollution plumes and environmental changes
- Citizen science programs engage the public in data collection
Big Data and Artificial Intelligence:
- Machine learning algorithms can predict pollution episodes
- Data mining techniques identify pollution sources and patterns
- Modeling systems simulate pollution transport and fate
Policy and Regulatory Trends
Environmental policy is evolving to address new challenges and incorporate scientific advances:
Science-Based Decision Making:
- Risk assessment frameworks that consider multiple endpoints
- Life cycle analysis to evaluate environmental impacts from cradle to grave
- Cumulative impact assessment that considers multiple stressors
Market-Based Approaches:
- Cap-and-trade systems for air pollutants
- Payment for ecosystem services programs
- Green chemistry incentives that reward safer chemical design
International Cooperation:
- Global plastic treaty negotiations to address marine plastic pollution
- Mercury Convention (Minamata Convention) to reduce mercury emissions
- Air quality cooperation in regions like East Asia and Europe
Study Guide Section: Mastering Unit 8 Concepts
Key Vocabulary and Definitions
Bioaccumulation: The accumulation of substances, such as pesticides or other chemicals, in an organism at concentrations greater than those in the surrounding environment.
Biomagnification: The increasing concentration of a substance, such as a toxic chemical, in the tissues of organisms at successively higher levels in a food chain.
Dead Zone: An area in a body of water where oxygen levels are so low that most marine life cannot survive, typically caused by eutrophication.
Eutrophication: The process by which water bodies become overly enriched with minerals and nutrients, leading to excessive growth of algae and depletion of oxygen.
Leachate: A liquid that has percolated through a solid and leached out some of the constituents, particularly referring to contaminated water from landfills.
Nonpoint Source Pollution: Pollution that comes from many diffuse sources rather than from a single point, such as agricultural runoff or urban stormwater.
Point Source Pollution: Pollution that comes from a single, identifiable source, such as a pipe, ditch, ship, or factory smokestack.
Thermal Pollution: The degradation of water quality by any process that changes ambient water temperature, typically from industrial cooling systems.
Essential Formulas and Calculations
Biochemical Oxygen Demand (BOD):
BOD measures the amount of oxygen required by microorganisms to decompose organic matter in water.
Higher BOD = More organic pollution = Less oxygen available for aquatic life
Dilution Calculation:
C₁V₁ = C₂V₂
Where: C₁ = initial concentration, V₁ = initial volume, C₂ = final concentration, V₂ = final volume
Dose Calculations:
Dose = (Concentration × Intake Rate × Exposure Duration) / Body Weight
Pollution Load:
Load = Concentration × Flow Rate
Often expressed as kg/day or tons/year
Study Strategies and Memory Aids
For Eutrophication Process:
Remember “NABO”: Nutrients → Algae → Bacteria → Oxygen depletion
For Types of Pollution:
Use “BPNT”: Biological, Physical, Nutrient, Toxic
For Pollution Transport:
Think “VASD”: Volatilization, Adsorption, Sedimentation, Degradation
For Waste Hierarchy:
“R³”: Reduce, Reuse, Recycle (in order of preference)
Common Exam Mistakes to Avoid
- Confusing bioaccumulation and biomagnification: Remember that bioaccumulation occurs within one organism, while biomagnification occurs across trophic levels.
- Forgetting about nonpoint source pollution: Students often focus on obvious point sources but forget that nonpoint sources cause most water pollution.
- Mixing up different types of treatment: Primary treatment removes solids, secondary treatment uses biological processes, tertiary treatment removes nutrients and other specific pollutants.
- Not considering environmental justice: Many pollution problems disproportionately affect marginalized communities.
Connecting to Other AP Environmental Science Units
- Unit 1 (The Living World): Pollution affects ecosystem structure and function
- Unit 2 (The Living World: Biodiversity): Pollution is a major driver of biodiversity loss
- Unit 3 (Populations): Pollution can affect population growth and carrying capacity
- Unit 4 (Earth Systems and Resources): Pollution cycles through air, water, and soil systems
- Unit 5 (Land and Water Use): Human activities that cause pollution
- Unit 6 (Energy Resources and Consumption): Energy production creates various forms of pollution
- Unit 7 (Atmospheric Pollution): Overlaps with air quality issues
- Unit 9 (Global Change): Pollution contributes to climate change and ozone depletion
Practice Questions: Test Your Knowledge
Multiple Choice Questions
- Which of the following best describes the difference between point source and nonpoint source pollution?
A) Point source pollution is more harmful than nonpoint source pollution
B) Point source pollution comes from a single identifiable location, while nonpoint source pollution comes from diffuse sources
C) Point source pollution only affects water, while nonpoint source pollution only affects land
D) Point source pollution is easier to clean up than nonpoint source pollution
E) Point source pollution is always chemical, while nonpoint source pollution is always biological - The process by which excess nutrients in water bodies lead to oxygen depletion is called:
A) Bioaccumulation
B) Biomagnification
C) Eutrophication
D) Thermal pollution
E) Acidification - Which of the following pollutants would most likely undergo biomagnification in a food chain?
A) Biodegradable organic matter
B) Thermal pollution
C) Excess nutrients (nitrogen and phosphorus)
D) Persistent organic pollutants like DDT
E) Sediments from erosion - The LD₅₀ value for a chemical represents:
A) The lowest dose that causes any observable effect
B) The dose that kills 50% of test organisms
C) The dose that causes effects in 50% of organisms
D) The highest dose that causes no adverse effects
E) The dose that bioaccumulates in 50% of organisms - Which of the following is NOT a characteristic of persistent organic pollutants (POPs)?
A) They resist environmental degradation
B) They can travel long distances through air and water
C) They readily dissolve in water
D) They accumulate in fatty tissues of organisms
E) They can biomagnify through food chains - The primary cause of dead zones in coastal waters is:
A) Oil spills from tanker accidents
B) Thermal pollution from power plants
C) Nutrient pollution leading to eutrophication
D) Heavy metal contamination from mining
E) Plastic pollution and marine debris - Which type of wastewater treatment removes nutrients like nitrogen and phosphorus?
A) Primary treatment
B) Secondary treatment
C) Tertiary treatment
D) Preliminary treatment
E) Disinfection treatment - Leachate from landfills is primarily a concern because it can:
A) Cause thermal pollution in nearby streams
B) Contaminate groundwater with toxic substances
C) Create dead zones in marine environments
D) Contribute to atmospheric ozone depletion
E) Cause acid rain in downwind areas - Which of the following best explains why environmental justice is a concern in pollution issues?
A) All pollution affects everyone equally regardless of income
B) Wealthy communities generate more pollution than poor communities
C) Poor and minority communities often bear disproportionate pollution burdens
D) Environmental laws only apply to certain demographic groups
E) Pollution cleanup is always funded by local communities - Microplastics are particularly concerning because they:
A) Only affect marine environments
B) Break down quickly in the environment
C) Are found throughout food webs and may carry toxic chemicals
D) Are only produced by industrial processes
E) Cannot travel long distances from their source
Free Response Questions
Question 1 (10 points): A coastal city is experiencing problems with eutrophication in its main bay. The bay receives nutrient inputs from agricultural runoff, urban stormwater, and a wastewater treatment plant.
(a) Describe the process of eutrophication, including the sequence of events from initial nutrient input to the formation of dead zones. (4 points)
(b) Explain how agricultural practices contribute to nutrient pollution and describe two specific best management practices that could reduce agricultural nutrient inputs to the bay. (3 points)
(c) Discuss the role of climate change in potentially worsening eutrophication problems and propose one policy solution that could address multiple pollution sources simultaneously. (3 points)
Question 2 (10 points): An industrial facility has been releasing heavy metals into a nearby river system for several decades before regulations required treatment of their wastewater.
(a) Explain the concepts of bioaccumulation and biomagnification, and describe how heavy metals would move through the aquatic food web in this river system. (4 points)
(b) Compare and contrast the environmental fate of heavy metals versus biodegradable organic pollutants in this river ecosystem. (3 points)
(c) Describe two different remediation technologies that could be used to address heavy metal contamination in the river sediments, and discuss the advantages and limitations of each approach. (3 points)
Answer Key and Explanations
Multiple Choice Answers:
- B – This is the fundamental definition distinguishing these pollution types
- C – Eutrophication is the classic process of nutrient enrichment leading to oxygen depletion
- D – POPs like DDT are fat-soluble and persistent, making them ideal for biomagnification
- B – LD₅₀ specifically refers to lethal dose for 50% of organisms
- C – POPs are typically fat-soluble, not water-soluble
- C – Nutrient pollution causing eutrophication is the primary cause of coastal dead zones
- C – Tertiary treatment specifically targets nutrients and other specific pollutants
- B – Leachate contamination of groundwater is the primary environmental concern from landfills
- C – Environmental justice focuses on disproportionate impacts on marginalized communities
- C – The widespread distribution and potential for carrying toxics makes microplastics particularly concerning
Free Response Sample Answers:
Question 1(a): Eutrophication begins when excess nutrients (nitrogen and phosphorus) enter water bodies from various sources. These nutrients act as fertilizers, stimulating rapid growth of algae and aquatic plants (algae blooms). When these organisms die, bacteria decompose them, consuming dissolved oxygen in the process. This creates hypoxic or anoxic conditions where fish and other aquatic life cannot survive, forming dead zones.
Question 1(b): Agricultural practices contribute through fertilizer application and animal waste. Excess fertilizers not taken up by crops wash off during rain events. Two BMPs: (1) Cover crops planted after harvest absorb residual nutrients and prevent runoff; (2) Riparian buffers consisting of vegetation strips along waterways filter nutrients from runoff before they reach water bodies.
Question 1(c): Climate change worsens eutrophication through increased temperatures (promoting algae growth), altered precipitation patterns (affecting nutrient transport), and stronger storms (increasing runoff). A watershed-based nutrient management plan with total maximum daily loads (TMDLs) could address all sources by setting limits and requiring coordinated reduction efforts across agriculture, urban areas, and point sources.
Conclusion and Next Steps: Your Journey in Environmental Science
Congratulations! You’ve now explored the complex world of aquatic and terrestrial pollution through the lens of AP Environmental Science. From the microscopic interactions of toxins with cellular processes to the global movement of persistent pollutants, you’ve gained the knowledge and analytical skills needed to understand one of our most pressing environmental challenges.
As you prepare for the AP exam, remember that pollution problems are rarely simple or isolated. They involve complex interactions between chemical, biological, and physical processes, and they often reflect broader social, economic, and political realities. The most effective solutions require interdisciplinary thinking and collaborative action-exactly the kind of systems thinking that makes environmental science such a vital field.
Key Takeaways for Success:
- Pollution is fundamentally about rates-when inputs exceed the environment’s capacity to process them
- Understanding pollution transport and fate helps predict impacts and design solutions
- Environmental justice considerations are integral to pollution problems and solutions
- Technology alone cannot solve pollution problems without addressing underlying behaviors and systems
- International cooperation is essential for addressing transboundary pollution issues
Your Role as an Environmental Citizen:
Armed with this knowledge, you’re now better equipped to:
- Make informed personal choices about consumption and waste
- Evaluate environmental policies and their potential effectiveness
- Participate in community discussions about local environmental issues
- Pursue further studies or careers in environmental science and related fields
The field of pollution science continues to evolve as we face new challenges like microplastics, emerging contaminants, and the interactions between pollution and climate change. Your generation will be at the forefront of developing innovative solutions to these complex problems.
Further Reading and Resources
To deepen your understanding of pollution science and environmental issues:
Essential References:
- EPA’s Pollution Prevention and Control Technologies Database (www.epa.gov) – Comprehensive information on treatment technologies and regulations
- National Institute of Environmental Health Sciences (www.niehs.nih.gov) – Research on health effects of environmental pollutants
- Stockholm Convention on Persistent Organic Pollutants (www.pops.int) – International treaty information and pollutant profiles
- Chesapeake Bay Program (www.chesapeakebay.net) – Real-world example of large-scale restoration efforts
- Environmental Justice Atlas (www.ejatlas.org) – Case studies of environmental conflicts and justice issues worldwide
Academic Journals for Advanced Study:
- Environmental Science & Technology
- Environmental Pollution
- Water Research
- Journal of Environmental Management
Remember, environmental science is ultimately about hope and action. Every pollution problem represents an opportunity to develop innovative solutions, build stronger communities, and create a more sustainable future. As you continue your studies and career, carry forward the systems thinking and scientific rigor you’ve developed, along with the passion for protecting our shared environment.
The future of our planet depends on informed, engaged citizens who understand the science behind environmental challenges and are committed to evidence-based solutions. You now have the foundation to be one of those leaders. Use it well!
Recommended –


Great post, I conceive blog owners should larn a lot from this website its very user friendly.