Why AP Chemistry Unit 4 Will Make or Break Your Exam Score
Picture this: You’re sitting in the AP Chemistry exam, and suddenly you encounter a complex stoichiometry problem involving multiple reaction types. Your heart races. This isn’t just another chemistry unit – AP Chemistry Unit 4: Chemical Reactions is the foundation upon which 25-30% of your entire AP exam score depends.
Whether you’re aiming for that coveted 5 or struggling to understand the difference between synthesis and decomposition reactions, this comprehensive guide will transform you from a confused student into a confident chemistry problem-solver.
The Problem Most Students Face: Chemical reactions seem like a maze of formulas, coefficients, and mysterious transformations. Many students memorize without understanding, leading to panic during exams when they encounter unfamiliar reaction scenarios.
Your Solution Starts Here: This guide breaks down every concept in AP Chemistry Unit 4 with real-world analogies, step-by-step problem-solving strategies, and insider exam tips that actual AP teachers use.
Reference: Your At-A-Glance Toolkit
Unit 4 Key Learning Objectives
- 4.1-4.3: Writing and balancing chemical equations
- 4.4-4.6: Stoichiometry and mole relationships
- 4.7-4.8: Reaction types and mechanisms
- 4.9-4.10: Physical vs. chemical changes
Essential Formulas & Constants
Mole Ratio = (coefficient of desired substance)/(coefficient of given substance)
Theoretical Yield = (limiting reactant moles) × (mole ratio) × (molar mass)
Percent Yield = (Actual Yield/Theoretical Yield) × 100%
Molarity = moles of solute/liters of solutionReaction Types Quick Identification
| Type | General Form | Memory Trick |
|---|---|---|
| Synthesis | A + B → AB | “Two become one” |
| Decomposition | AB → A + B | “One becomes many” |
| Single Replacement | A + BC → AC + B | “One element switches places” |
| Double Replacement | AB + CD → AD + CB | “Partners swap dance partners” |
Common Ion Charges (Must Memorize!)
- +1: Li⁺, Na⁺, K⁺, NH₄⁺, Ag⁺
- +2: Mg²⁺, Ca²⁺, Ba²⁺, Zn²⁺, Cd²⁺
- -1: F⁻, Cl⁻, Br⁻, I⁻, OH⁻, NO₃⁻
- -2: O²⁻, S²⁻, SO₄²⁻, CO₃²⁻
Foundation Concepts: Building Your Chemistry Fortress
Understanding Chemical Equations: The Language of Chemistry
Think of chemical equations as recipes. Just like you wouldn’t add 5 cups of flour when a recipe calls for 1 cup, chemical reactions require precise ratios to work properly.
The Anatomy of a Chemical Equation:
2H₂ + O₂ → 2H₂O- Reactants: H₂ and O₂ (ingredients)
- Products: H₂O (final dish)
- Coefficients: 2, 1, 2 (quantities needed)
- Arrow: Shows direction of reaction
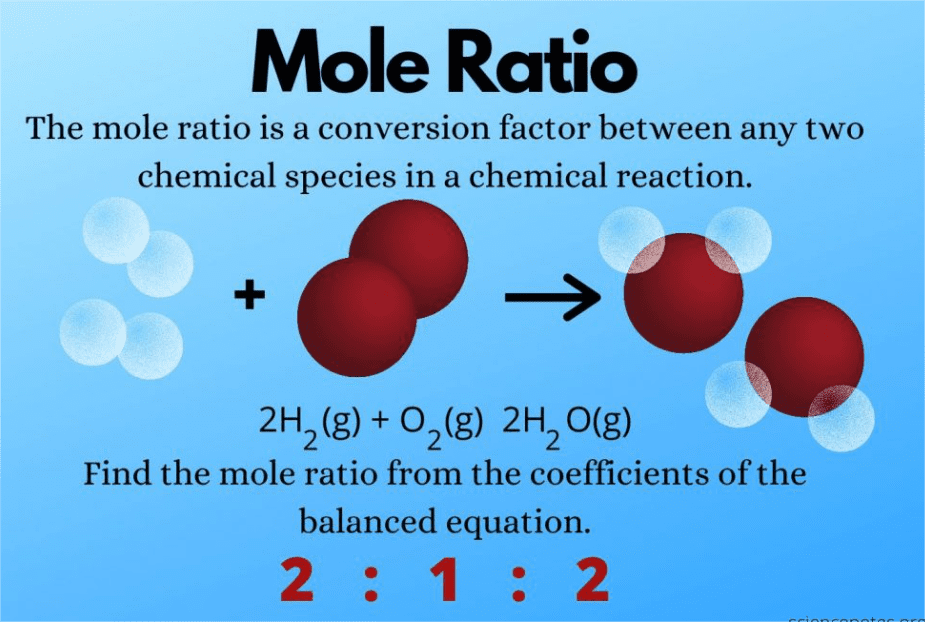
The Mole: Your Universal Chemistry Currency
Imagine the mole as chemistry’s universal currency – like how different countries use different money, but we can convert between them. One mole always equals 6.022 × 10²³ particles, whether they’re atoms, molecules, or ions.
Real-World Analogy: Just like a dozen always means 12 (whether it’s 12 eggs or 12 cars), a mole always means 6.022 × 10²³ particles.
Balancing Chemical Equations: The Art of Chemical Bookkeeping
Step-by-Step Method:
- Count atoms of each element on both sides
- Start with the most complex molecule
- Balance metals first, then non-metals, then hydrogen and oxygen
- Use whole number coefficients only
- Check your work – atoms on left = atoms on right
Practice Example:
Unbalanced: Fe + O₂ → Fe₂O₃
Step 1: Fe atoms: 1 left, 2 right
Step 2: O atoms: 2 left, 3 right
Step 3: Try coefficients: 4Fe + 3O₂ → 2Fe₂O₃
Step 4: Check: Fe: 4 = 4 , O: 6 = 6 Deep Dive: The Four Pillars of Chemical Reactions
1. Synthesis Reactions (Combination): Building Complexity
General Pattern: A + B → AB
These reactions are like construction projects – simple materials combine to create something more complex.
Real-World Examples:
- Rusting: 4Fe + 3O₂ → 2Fe₂O₃
- Photosynthesis: 6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
- Salt Formation: Na + Cl₂ → 2NaCl
Key Insight: Energy is usually released (exothermic) because bonds forming release more energy than bonds breaking.
2. Decomposition Reactions: Breaking Things Apart
General Pattern: AB → A + B
Think of these as demolition projects – complex substances break down into simpler components.
Common Triggers:
- Heat: CaCO₃ → CaO + CO₂ (limestone decomposition)
- Electricity: 2H₂O → 2H₂ + O₂ (electrolysis)
- Light: 2AgBr → 2Ag + Br₂ (photography)
Key Insight: Energy is usually required (endothermic) to break bonds.
3. Single Replacement: The Chemistry of Competition
General Pattern: A + BC → AC + B
Imagine this as a competitive sport where a more reactive element “kicks out” a less reactive one.
Activity Series (Most to Least Reactive):
Li > K > Ba > Ca > Na > Mg > Al > Zn > Fe > Ni > Sn > Pb > H > Cu > Ag > Au
Prediction Rule: An element can only replace another element below it in the activity series.
Example:
Zn + CuSO₄ → ZnSO₄ + Cu (Zn is above Cu)
Cu + ZnSO₄ → No Reaction (Cu is below Zn)4. Double Replacement: The Great Partner Swap
General Pattern: AB + CD → AD + CB
Think of this as a dance where partners switch – like two couples swapping partners.
Three Types of Double Replacement:
A) Precipitation Reactions
- Form an insoluble solid (precipitate)
- Example: AgNO₃ + NaCl → AgCl↓ + NaNO₃
B) Acid-Base Neutralization
- Form water and a salt
- Example: HCl + NaOH → H₂O + NaCl
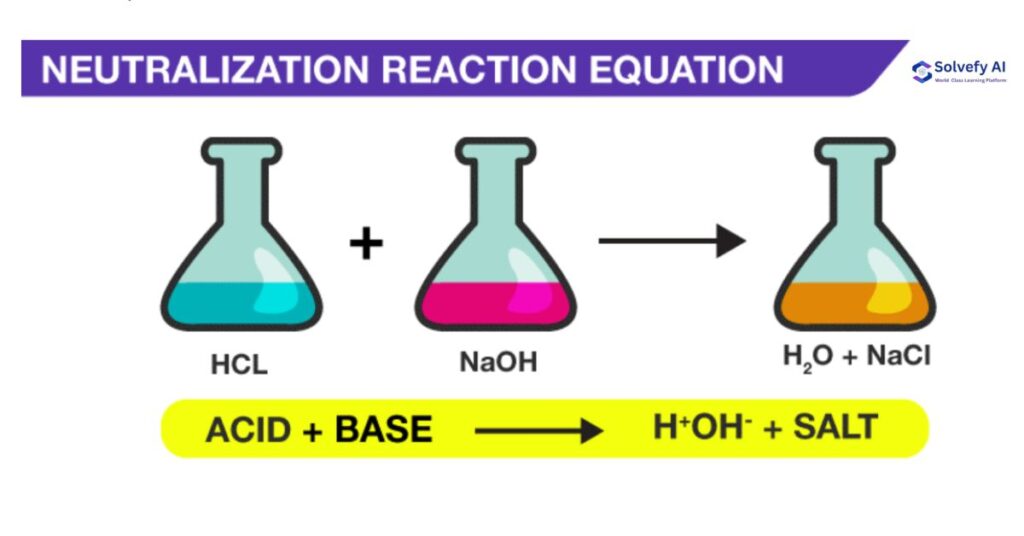
C) Gas Formation
- Produce a gaseous product
- Example: HCl + NaHCO₃ → H₂O + CO₂↑ + NaCl
Stoichiometry Mastery: Your Mathematical Superpower
The Stoichiometry Roadmap
Think of stoichiometry as GPS for chemistry – it tells you exactly how to get from Point A (what you have) to Point B (what you want).
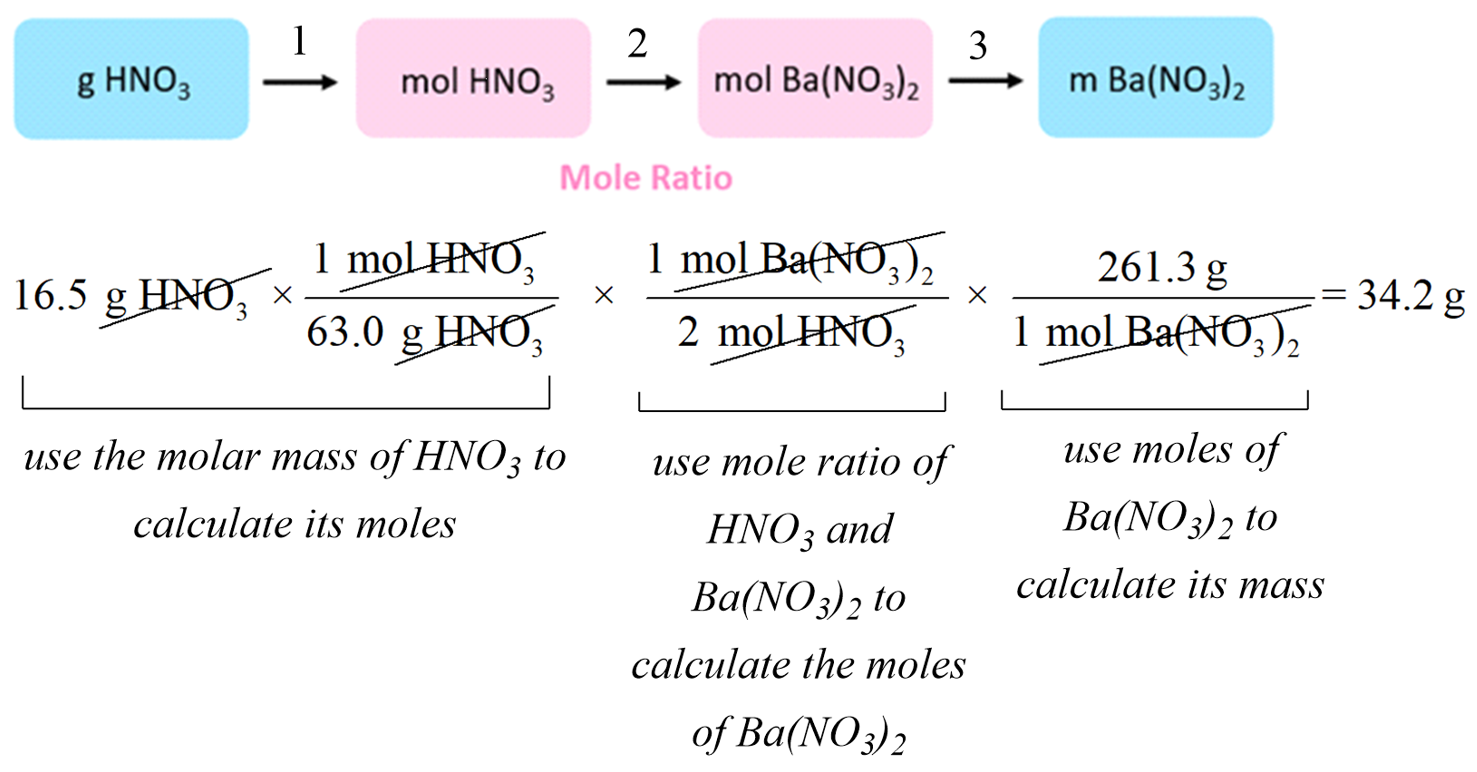
The Universal Conversion Path:
Given → Moles Given → Moles Wanted → AnswerStep-by-Step Stoichiometry Strategy
Example Problem: How many grams of CO₂ are produced when 5.0 g of C₃H₈ burns completely?
Step 1: Write the balanced equation
C₃H₈ + 5O₂ → 3CO₂ + 4H₂OStep 2: Convert given to moles
5.0 g C₃H₈ × (1 mol C₃H₈/44.1 g C₃H₈) = 0.113 mol C₃H₈Step 3: Use mole ratio
0.113 mol C₃H₈ × (3 mol CO₂/1 mol C₃H₈) = 0.340 mol CO₂Step 4: Convert to desired units
0.340 mol CO₂ × (44.0 g CO₂/1 mol CO₂) = 15.0 g CO₂Limiting Reactant Problems: The Chemistry Bottleneck
Real-World Analogy: Making sandwiches with 10 pieces of bread and 3 slices of cheese. The cheese limits how many sandwiches you can make (only 3), even though you have extra bread.
Strategy:
- Calculate moles of each reactant
- Determine theoretical yield from each reactant
- The smallest yield identifies the limiting reactant
- Use limiting reactant for final calculations
15 Multiple Choice Practice Questions with Detailed Solutions
Questions 1-5: Reaction Types and Balancing
1. Which coefficients correctly balance the equation: ___Al + ___O₂ → ___Al₂O₃?
A) 2, 3, 1
B) 4, 3, 2
C) 1, 1, 1
D) 3, 2, 1
Answer: B) 4, 3, 2
Detailed Explanation:
- Left side: 4 Al atoms, 6 O atoms (3 × 2)
- Right side: 4 Al atoms (2 × 2), 6 O atoms (2 × 3)
- The equation balances: 4Al + 3O₂ → 2Al₂O₃
2. The reaction 2KClO₃ → 2KCl + 3O₂ is an example of:
A) Synthesis
B) Decomposition
C) Single replacement
D) Double replacement
Answer: B) Decomposition
Detailed Explanation: One compound (KClO₃) breaks down into two simpler substances (KCl and O₂). This fits the pattern AB → A + B.
3. In the reaction Zn + 2HCl → ZnCl₂ + H₂, what is the mole ratio of Zn to H₂?
A) 1:1
B) 1:2
C) 2:1
D) 2:2
Answer: A) 1:1
Detailed Explanation: From the balanced equation, 1 mole of Zn produces 1 mole of H₂. The coefficients give us the mole ratio directly.
4. Which reaction will definitely produce a precipitate?
A) NaCl + KBr →
B) AgNO₃ + NaCl →
C) NaOH + HCl →
D) CaCl₂ + NaNO₃ →
Answer: B) AgNO₃ + NaCl →
Detailed Explanation: This produces AgCl, which is insoluble according to solubility rules (most chlorides are soluble, but AgCl is an exception).
5. The percent yield of a reaction is 85%. If the theoretical yield is 12.0 g, what is the actual yield?
A) 10.2 g
B) 10.8 g
C) 14.1 g
D) 9.6 g
Answer: A) 10.2 g
Detailed Explanation:
Actual yield = (% yield/100) × theoretical yield
Actual yield = (85/100) × 12.0 g = 10.2 g
Questions 6-10: Stoichiometry and Mole Calculations
6. How many moles of O₂ are needed to completely react with 0.50 moles of C₂H₆ in the reaction: 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O?
A) 1.75 mol
B) 3.5 mol
C) 0.25 mol
D) 7.0 mol
Answer: A) 1.75 mol
Detailed Explanation:
Mole ratio: 7 mol O₂ : 2 mol C₂H₆
0.50 mol C₂H₆ × (7 mol O₂/2 mol C₂H₆) = 1.75 mol O₂
7. In the reaction 2A + 3B → C, if 4.0 moles of A and 3.0 moles of B are mixed, which is the limiting reactant?
A) A
B) B
C) C
D) Neither
Answer: B) B
Detailed Explanation:
From A: 4.0 mol A × (1 mol C/2 mol A) = 2.0 mol C possible
From B: 3.0 mol B × (1 mol C/3 mol B) = 1.0 mol C possible
B produces less product, so B is limiting.
8. What mass of CO₂ is produced when 22.0 g of propane (C₃H₈) burns completely?
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O (Molar mass C₃H₈ = 44.1 g/mol, CO₂ = 44.0 g/mol)
A) 22.0 g
B) 44.0 g
C) 66.0 g
D) 132 g
Answer: C) 66.0 g
Detailed Explanation:
22.0 g C₃H₈ × (1 mol C₃H₈/44.1 g) × (3 mol CO₂/1 mol C₃H₈) × (44.0 g CO₂/1 mol CO₂) = 66.0 g CO₂
9. If 2.5 L of 0.10 M HCl reacts with excess NaOH, how many moles of water are formed?
A) 0.025 mol
B) 0.25 mol
C) 2.5 mol
D) 25 mol
Answer: B) 0.25 mol
Detailed Explanation:
Moles HCl = 0.10 M × 2.5 L = 0.25 mol
HCl + NaOH → H₂O + NaCl (1:1 ratio)
Therefore, 0.25 mol H₂O formed.
10. In a combustion reaction, if 95% of the carbon forms CO₂ and 5% forms CO, this affects:
A) The balancing of the equation
B) The theoretical yield calculation
C) The limiting reactant determination
D) All of the above
Answer: D) All of the above
Detailed Explanation: Incomplete combustion changes the products, affecting equation balancing, yield calculations, and reactant consumption ratios.
Questions 11-15: Advanced Applications
11. Which factor does NOT affect reaction rate?
A) Temperature
B) Concentration
C) Catalyst presence
D) Theoretical yield
Answer: D) Theoretical yield
Detailed Explanation: Theoretical yield is a calculated value based on stoichiometry and doesn’t influence how fast a reaction proceeds.
12. The reaction 2NO + O₂ → 2NO₂ has a rate law: Rate = k[NO]²[O₂]. If [NO] doubles and [O₂] triples, the rate:
A) Doubles
B) Triples
C) Increases 12-fold
D) Increases 6-fold
Answer: C) Increases 12-fold
Detailed Explanation:
New rate = k[2NO]²[3O₂] = k(4)[NO]²(3)[O₂] = 12k[NO]²[O₂]
The rate increases by a factor of 12.
13. In a titration, 25.0 mL of 0.100 M NaOH neutralizes 20.0 mL of HCl. What is the molarity of the HCl?
A) 0.080 M
B) 0.125 M
C) 0.100 M
D) 0.200 M
Answer: B) 0.125 M
Detailed Explanation:
Moles NaOH = 0.100 M × 0.0250 L = 0.00250 mol
Moles HCl = moles NaOH = 0.00250 mol (1:1 ratio)
Molarity HCl = 0.00250 mol / 0.0200 L = 0.125 M
14. Which compound is most likely to be ionic?
A) CO₂
B) CH₄
C) CaF₂
D) NH₃
Answer: C) CaF₂
Detailed Explanation: CaF₂ is formed between a metal (Ca) and a nonmetal (F), showing the largest electronegativity difference, making it ionic.
15. The equilibrium constant expression for 2A + B ⇌ 3C + D is:
A) K = [C]³[D]/[A]²[B]
B) K = [A]²[B]/[C]³[D]
C) K = 3[C][D]/2[A][B]
D) K = [C][D]/[A][B]
Answer: A) K = [C]³[D]/[A]²[B]
Detailed Explanation: The equilibrium expression has products in the numerator and reactants in the denominator, each raised to the power of their coefficients.
3 Free Response Practice Problems with Step-by-Step Solutions
Problem 1: Comprehensive Stoichiometry (8 points)
The combustion of methanol (CH₃OH) produces carbon dioxide and water according to the following unbalanced equation:
CH₃OH + O₂ → CO₂ + H₂O
(a) Balance the chemical equation. (1 point)
Solution:
2CH₃OH + 3O₂ → 2CO₂ + 4H₂O
Balancing process:
- C: 2 left = 2 right ✓
- H: 8 left = 8 right ✓
- O: 8 left = 8 right ✓
(b) If 64.0 g of methanol reacts with excess oxygen, calculate the theoretical yield of CO₂ in grams. (3 points)
Solution:
Step 1: Convert mass to moles
Molar mass CH₃OH = 32.0 g/mol
64.0 g CH₃OH × (1 mol CH₃OH/32.0 g CH₃OH) = 2.00 mol CH₃OH
Step 2: Use stoichiometry
2.00 mol CH₃OH × (2 mol CO₂/2 mol CH₃OH) = 2.00 mol CO₂
Step 3: Convert to mass
Molar mass CO₂ = 44.0 g/mol
2.00 mol CO₂ × (44.0 g CO₂/1 mol CO₂) = 88.0 g CO₂
(c) If the actual yield is 79.2 g, what is the percent yield? (2 points)
Solution:
Percent yield = (actual yield/theoretical yield) × 100%
Percent yield = (79.2 g/88.0 g) × 100% = 90.0%
(d) Suggest two reasons why the actual yield might be less than theoretical yield. (2 points)
Solution:
- Incomplete reaction – Not all reactants may have been converted to products
- Side reactions – Some methanol may have undergone different reactions producing other products
- Product loss during purification – Some CO₂ may have escaped during collection
Problem 2: Limiting Reactant Analysis (7 points)
Iron(III) oxide reacts with carbon monoxide to produce iron and carbon dioxide:
Fe₂O₃ + 3CO → 2Fe + 3CO₂
A mixture contains 15.0 g of Fe₂O₃ and 12.0 g of CO.
(a) Determine the limiting reactant. Show all calculations. (4 points)
Solution:
Step 1: Calculate moles of each reactant
Molar mass Fe₂O₃ = 159.7 g/mol
15.0 g Fe₂O₃ × (1 mol Fe₂O₃/159.7 g Fe₂O₃) = 0.0939 mol Fe₂O₃
Molar mass CO = 28.0 g/mol
12.0 g CO × (1 mol CO/28.0 g CO) = 0.429 mol CO
Step 2: Calculate theoretical yield from each reactant
From Fe₂O₃: 0.0939 mol Fe₂O₃ × (2 mol Fe/1 mol Fe₂O₃) = 0.188 mol Fe
From CO: 0.429 mol CO × (2 mol Fe/3 mol CO) = 0.286 mol Fe
Step 3: Identify limiting reactant
Fe₂O₃ produces less Fe (0.188 mol vs 0.286 mol), so Fe₂O₃ is the limiting reactant.
(b) Calculate the mass of iron produced. (2 points)
Solution:
0.188 mol Fe × (55.8 g Fe/1 mol Fe) = 10.5 g Fe
(c) How many grams of the excess reactant remain unreacted? (1 point)
Solution:
CO needed: 0.0939 mol Fe₂O₃ × (3 mol CO/1 mol Fe₂O₃) = 0.282 mol CO
CO excess: 0.429 mol – 0.282 mol = 0.147 mol CO
Mass excess: 0.147 mol CO × (28.0 g CO/1 mol CO) = 4.12 g CO
Problem 3: Precipitation and Net Ionic Equations (6 points)
When solutions of lead(II) nitrate and potassium iodide are mixed, a yellow precipitate forms.
(a) Write the balanced molecular equation. (1 point)
Solution:
Pb(NO₃)₂ + 2KI → PbI₂ + 2KNO₃
(b) Write the complete ionic equation. (2 points)
Solution:
Pb²⁺ + 2NO₃⁻ + 2K⁺ + 2I⁻ → PbI₂(s) + 2K⁺ + 2NO₃⁻
(c) Write the net ionic equation. (1 point)
Solution:
Pb²⁺ + 2I⁻ → PbI₂(s)
(d) If 25.0 mL of 0.150 M Pb(NO₃)₂ reacts with 30.0 mL of 0.200 M KI, calculate the mass of precipitate formed. (2 points)
Solution:
Step 1: Calculate moles of reactants
Moles Pb(NO₃)₂ = 0.150 M × 0.0250 L = 0.00375 mol
Moles KI = 0.200 M × 0.0300 L = 0.00600 mol
Step 2: Determine limiting reactant
Need 2 mol KI per 1 mol Pb(NO₃)₂
KI needed = 0.00375 mol × 2 = 0.00750 mol
Since only 0.00600 mol KI available, KI is limiting
Step 3: Calculate PbI₂ formed
0.00600 mol KI × (1 mol PbI₂/2 mol KI) = 0.00300 mol PbI₂
Mass = 0.00300 mol × 461.0 g/mol = 1.38 g PbI₂
Study Strategies and Exam Preparation Tips
The 4-Phase Mastery Plan
Phase 1: Foundation Building (Week 1-2)
- Master basic reaction types using flashcards
- Practice balancing equations for 15 minutes daily
- Memorize common ions and their charges
- Learn solubility rules using mnemonics
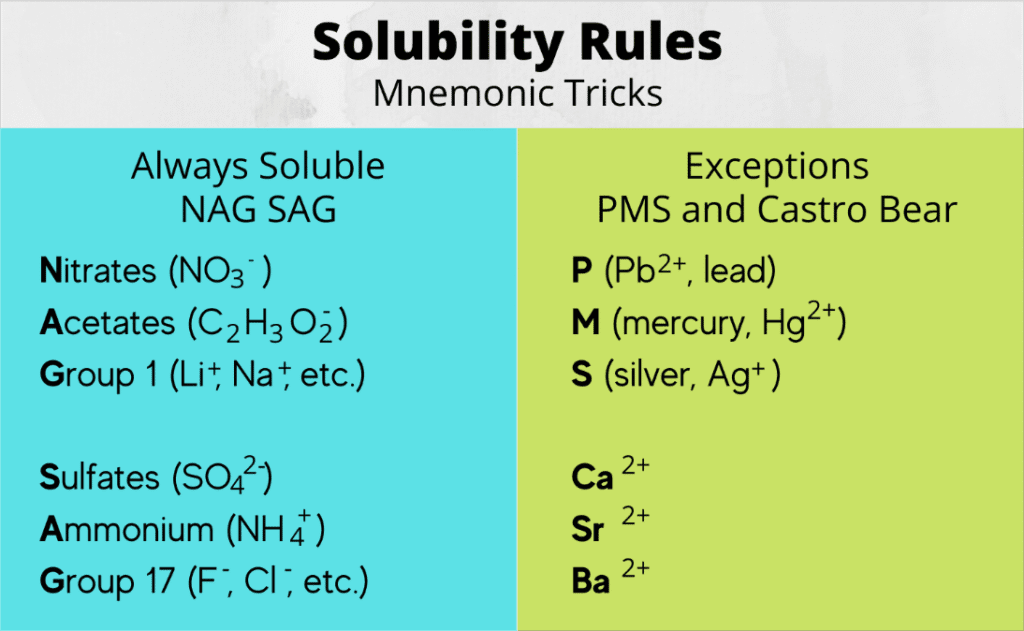
Memory Trick for Solubility:
“NAG SAP” – Nitrates, Acetates, Group 1, Sulfates (except BaSO₄, PbSO₄), Ammonium, Perchlorates are generally soluble.
Phase 2: Skill Development (Week 3-4)
- Daily stoichiometry problems (start with 3, build to 10)
- Practice limiting reactant scenarios
- Work through precipitation problems
- Time yourself on basic calculations
Phase 3: Integration and Speed (Week 5-6)
- Mixed problem sets combining multiple concepts
- Timed practice tests (45 minutes for 15 MC questions)
- Error analysis – keep a mistake journal
- Teach concepts to classmates or family
Phase 4: Mastery and Refinement (Week 7-8)
- Full-length practice exams
- Review common mistakes
- Perfect your FRQ format
- Final concept reinforcement
Time Management Strategies for the AP Exam
Multiple Choice Section (90 minutes, 60 questions)
- 1.5 minutes per question average
- Skip and return to difficult problems
- Eliminate obviously wrong answers first
- Use dimensional analysis for unit problems
Free Response Section (105 minutes, 7 questions)
- 15 minutes per question average
- Read all questions first (2 minutes)
- Start with your strongest topic
- Show all work clearly
- Leave partial answers rather than blank responses
The “PASS” Strategy for Difficult Problems:
- Pause and breathe
- Analyze what’s given and what’s asked
- Simplify using basic principles
- Show your work even if uncertain
Common Error Analysis and Prevention
Top 5 Student Mistakes:
1. Coefficient vs. Subscript Confusion
- Wrong: Changing H₂O to H₃O to balance
- Right: Using coefficients: 2H₂O
2. Forgetting to Balance Equations
- Wrong: Using unbalanced equations in stoichiometry
- Right: Always balance first, then calculate
3. Unit Conversion Errors
- Wrong: Mixing grams and kilograms
- Right: Check units at every step
4. Limiting Reactant Shortcuts
- Wrong: Assuming the smaller amount is limiting
- Right: Calculate theoretical yields from both reactants
5. Rounding Too Early
- Wrong: Rounding at each step
- Right: Keep extra digits until the final answer
Real-World Applications and Connections
Industrial Chemistry: Where Theory Meets Reality
Haber Process – Feeding the World
N₂ + 3H₂ ⇌ 2NH₃This reaction produces ammonia for fertilizers that feed nearly half the world’s population. The stoichiometry principles you’re learning help optimize conditions for maximum yield.
Steel Production – Building Civilization
Fe₂O₃ + 3CO → 2Fe + 3CO₂The same limiting reactant concepts you practice determine how much iron can be extracted from ore, affecting everything from skyscrapers to smartphones.
Environmental Chemistry: Solving Global Challenges
Carbon Capture and Storage
CO₂ + CaO → CaCO₃Understanding precipitation reactions helps design systems to remove CO₂ from the atmosphere, combating climate change.
Wastewater Treatment
Al₂(SO₄)₃ + 6NaOH → 2Al(OH)₃ + 3Na₂SO₄Double replacement reactions remove pollutants from water, protecting our ecosystems.
Medical Applications: Chemistry Saving Lives
Antacid Reactions
CaCO₃ + 2HCl → CaCl₂ + H₂O + CO₂The acid-base neutralization you study happens in your stomach when you take antacids.
Drug Synthesis
Complex organic syntheses use the same stoichiometric principles to ensure correct drug dosages and minimize side effects.
Energy Storage and Batteries
Lithium-Ion Battery Reactions
LiCoO₂ + C₆ ⇌ Li₁₋ₓCoO₂ + LiₓC₆The electron transfer and stoichiometry concepts determine battery capacity and charging efficiency.
FAQs: Your Concerns Answered
Q1: “How do I know which reaction type I’m dealing with?”
Answer: Use this decision tree:
- One product? → Synthesis
- One reactant? → Decomposition
- Element + compound? → Single replacement
- Two compounds switching parts? → Double replacement
Pro Tip: Practice with 20-30 examples until pattern recognition becomes automatic.
Q2: “I always mess up stoichiometry calculations. Help!”
Answer: Follow the “GRAMS → MOLES → MOLES → GRAMS” pathway religiously:
- Always convert to moles first
- Use balanced equation for ratios
- Convert back to desired units
- Check units at every step
Memory Device: “Good Mathematicians Make Money” (Grams → Moles → Moles → Money/Answer)
Q3: “How can I memorize all the solubility rules?”
Answer: Use the “NAGS + exceptions” method:
- Nitrates – all soluble
- Acetates – all soluble
- Group 1 – all soluble
- Sulfates – soluble except Ba²⁺, Sr²⁺, Pb²⁺
For other ions, focus on the common insoluble ones: carbonates, phosphates, hydroxides (except Group 1 and Ba²⁺).
Q4: “What’s the difference between theoretical and actual yield?”
Answer:
- Theoretical yield = Perfect world calculation (100% efficiency)
- Actual yield = What you really get in the lab
- Percent yield = (Actual/Theoretical) × 100%
Real-world factors affecting yield: side reactions, incomplete reactions, product loss during purification, measurement errors.
Q5: “How do I approach limiting reactant problems without getting confused?”
Answer: Use the “Two-Path Method”:
- Calculate product from Reactant A
- Calculate product from Reactant B
- Smaller amount identifies limiting reactant
- Use limiting reactant for final answer
Analogy: Like making sandwiches – if you have 10 pieces of bread and 3 slices of cheese, cheese limits you to 3 sandwiches.
Q6: “I panic during timed exams. Any advice?”
Answer: Practice the “Breathe-Read-Plan-Execute” method:
- Take 3 deep breaths when you feel overwhelmed
- Read the question twice before starting
- Plan your approach (what formulas/concepts needed?)
- Execute systematically with clear units
Remember: Partial credit is better than no credit. Show your thinking process.
Q7: “Should I memorize every possible reaction?”
Answer: No! Focus on patterns and principles:
- Understand the 4 main types deeply
- Learn prediction rules (activity series, solubility rules)
- Practice recognizing patterns rather than memorizing specific reactions
- Master the underlying concepts that apply to all reactions
Q8: “How important is Unit 4 for the overall AP exam?”
Answer: Extremely important! Unit 4 concepts appear in:
- 25-30% of multiple choice questions directly
- Foundation for Units 5-9 (thermodynamics, kinetics, equilibrium)
- Most free response questions require stoichiometry
- Laboratory scenarios often involve reaction analysis
Bottom line: Master Unit 4, and you set yourself up for success throughout the entire course.
Comprehensive Conclusion and Your Next Steps
What You’ve Accomplished
Congratulations! You’ve just completed a comprehensive journey through AP Chemistry Unit 4: Chemical Reactions. You now have:
✅ Solid foundation in all four reaction types
✅ Mastery tools for stoichiometry calculations
✅ Strategic approach to limiting reactant problems
✅ Practical experience with 15 multiple choice questions
✅ Deep understanding through 3 detailed FRQ solutions
✅ Study strategies proven to boost exam scores
✅ Real-world connections that make chemistry meaningful
✅ Confidence-building through systematic error prevention
Your Immediate Action Plan
This Week:
- Review your mistake journal from practice problems
- Create flashcards for reaction types and formulas
- Practice 5 stoichiometry problems daily
- Quiz yourself on solubility rules
Next Week:
- Take a timed practice test (45 minutes, 15 questions)
- Analyze your performance using the error patterns discussed
- Focus extra time on your weakest areas
- Join or form a study group for peer teaching
Before Your Next Exam:
- Complete 2 full-length practice tests
- Review all FRQ solutions in this guide
- Perfect your time management strategies
- Get adequate sleep and nutrition
Building Toward AP Chemistry Mastery
Unit 4 sets the foundation for:
- Unit 5: Kinetics – How fast reactions occur
- Unit 6: Thermodynamics – Energy changes in reactions
- Unit 7: Equilibrium – When reactions reach balance
- Unit 8: Acids and Bases – Special types of reactions
- Unit 9: Applications – Putting it all together
The concepts you’ve mastered here will appear again and again throughout your AP Chemistry journey.
Remember: You’ve Got This!
Every chemistry expert was once where you are now – facing complex equations, wondering how to balance reactions, and feeling overwhelmed by stoichiometry. The difference between struggling students and successful ones isn’t natural ability; it’s consistent practice, strategic study methods, and refusing to give up.
Your chemistry journey doesn’t end here – it transforms. The skills you’ve built in Unit 4 will serve you not just in AP Chemistry, but in:
- College chemistry courses
- STEM careers in medicine, engineering, and research
- Critical thinking skills for any profession
- Problem-solving approaches that apply far beyond chemistry
Stay Connected and Keep Learning
Chemistry is everywhere around you. Every time you:
- Cook food (chemical reactions)
- Take medicine (stoichiometry and dosing)
- Start your car (combustion reactions)
- Charge your phone (electrochemical reactions)
…you’re witnessing the principles you’ve just mastered.
Final Challenge: Choose one real-world chemical process that interests you and analyze it using Unit 4 concepts. Share your findings with classmates or family. Teaching others is the ultimate test of your understanding.
You’re Not Just Learning Chemistry – You’re Becoming a Scientist
The systematic thinking, attention to detail, and problem-solving persistence you’ve developed will serve you throughout your academic and professional career. Whether you become a doctor saving lives, an engineer building sustainable technologies, or a teacher inspiring the next generation, these skills are your foundation.
The AP Chemistry exam is just one milestone in your scientific journey. The real reward is the powerful way of thinking you’re developing – the ability to look at complex problems, break them into manageable parts, and find elegant solutions.
Keep practicing, stay curious, and remember: every expert was once a beginner who refused to give up.
Also Read –


2 thoughts on “AP Chemistry Unit 4: Chemical Reactions – The Ultimate 2025 Study Guide & Review”