Picture this: You’re sipping your morning coffee, scrolling through social media, when you suddenly realize that the caffeine molecules binding to receptors in your brain, the glucose from your breakfast fueling your cells, and even the DNA in every cell of your body all follow the same fundamental chemical principles you’re about to master in AP Biology Unit 1. Welcome to the Chemistry of Life – where the microscopic world of atoms and molecules creates the foundation for everything that makes you… you.
If you’ve ever wondered how your body manages to coordinate trillions of chemical reactions every second, or how a simple change in pH can mean the difference between life and death, you’re in the right place. Unit 1 isn’t just about memorizing molecular structures (though we’ll definitely cover those) – it’s about understanding the elegant chemical choreography that makes life possible.
What You’ll Master by the End of This Guide
By the time you finish this comprehensive guide, you’ll be able to:
- Explain how the properties of water make life on Earth possible
- Describe the structure and function of biological macromolecules
- Analyze how enzymes catalyze biochemical reactions
- Predict how changes in environmental conditions affect biological systems
- Connect molecular-level processes to organism-level functions
- Tackle any AP Biology exam question related to biochemistry with confidence
Quick Check Box: Before We Begin
Rate your current understanding (1-5 scale):
- Water’s unique properties: –
- Protein structure and function: –
- Enzyme kinetics: –
- Carbohydrate types and roles: –
- We’ll get all of these to a 5 by the end!
The Molecular Foundation: Why Chemistry Matters in Biology
Before diving into the specifics, let’s address the elephant in the room: “Why do I need to know chemistry to understand biology?” Here’s the thing – biology IS chemistry, just chemistry that happens to be alive. Every biological process, from photosynthesis in plants to the neural firing in your brain right now, follows the same chemical laws that govern non-living matter.
Think about it this way: if biology were a house, chemistry would be the foundation. You can’t understand how the house functions without understanding what it’s built on. This is why the College Board starts AP Biology with chemistry – not to torture you with molecular formulas, but to give you the tools to understand everything else that comes after.
Real-World Connection: The $3.8 Billion Industry
The global enzyme market is projected to reach $3.8 billion by 2025, with applications in everything from laundry detergent to cancer treatment. Understanding enzyme chemistry isn’t just academic – it’s the foundation of biotechnology careers that didn’t exist a generation ago.
Water: The Ultimate Biological Solvent
Let’s start with the most abundant molecule in your body – water. You’re roughly 60% water, but H₂O isn’t just filling space. Water’s unique chemical properties make it the perfect medium for life, and understanding these properties is crucial for AP Biology success.
The Polar Nature of Water: Why Opposites Attract
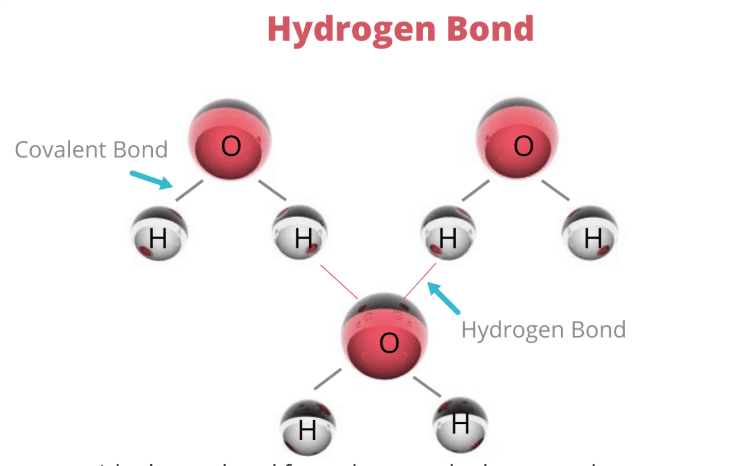
Water’s magic begins with its molecular structure. The oxygen atom hoards electrons more than the hydrogen atoms, creating a molecule with a slightly negative end (oxygen) and slightly positive ends (hydrogens). This polarity is like having a tiny magnet in every water molecule.
But here’s where it gets interesting for biological systems: this polarity allows water molecules to form hydrogen bonds with each other and with other polar substances. These aren’t as strong as covalent bonds, but they’re strong enough to give water its life-supporting properties.
Study Tip: The Hydrogen Bond Memory Trick
Remember “HONF” – Hydrogen bonds form when H is attached to Oxygen, Nitrogen, or Fluorine. For AP Biology, focus on H-O and H-N interactions, as these are most common in biological systems.
Water’s Life-Supporting Properties
1. High Specific Heat Capacity
Water can absorb lots of heat energy before its temperature rises significantly. This means your body temperature stays relatively stable even when your metabolism ramps up during exercise. On a larger scale, oceans moderate Earth’s climate because they can store enormous amounts of thermal energy.
AP Exam Insight: Questions often ask you to predict what would happen to organisms if water had a lower specific heat. The answer: rapid, dangerous temperature fluctuations that would denature proteins.
2. High Heat of Vaporization
It takes a lot of energy to convert liquid water to water vapor. This is why sweating is such an effective cooling mechanism – when sweat evaporates, it carries away significant heat energy, cooling your body.
3. Cohesion and Adhesion
Water molecules stick to each other (cohesion) and to other surfaces (adhesion). This creates surface tension and allows plants to transport water from roots to leaves through incredibly thin tubes called xylem. Without these properties, trees couldn’t exist as we know them.
4. Universal Solvent Properties
Water dissolves more substances than any other liquid, earning it the nickname “universal solvent.” This allows cells to transport nutrients, waste products, and signaling molecules dissolved in aqueous solutions.
Common Mistake Alert: Hydrophobic vs. Hydrophilic
Students often confuse these terms. Remember: Hydrophilic = “water-loving” (polar substances that dissolve in water). Hydrophobic = “water-fearing” (nonpolar substances that don’t dissolve in water). Cell membranes exist because phospholipids have both hydrophilic heads and hydrophobic tails.
Ice: The Floating Exception
Unlike most substances, water expands when it freezes. This means ice is less dense than liquid water and floats. This seemingly simple fact has profound biological implications – ice forms an insulating layer on top of bodies of water, allowing aquatic life to survive underneath during winter.
Quick Check: Water Properties
Can you explain why each of these scenarios depends on water’s unique properties?
- A water strider walking on a pond’s surface
- Your ability to cool down by sweating
- Fish surviving in a frozen pond
- Salt dissolving in your bloodstream
The pH Scale: Life in the Balance
Now that you understand water’s structure, let’s explore one of its most biologically important properties – its ability to ionize. Water molecules occasionally break apart into H⁺ (hydrogen) and OH⁻ (hydroxide) ions. The concentration of these ions determines pH, and small changes in pH can have massive biological consequences.
Understanding pH: More Than Just Numbers
The pH scale ranges from 0-14, with 7 being neutral. But here’s what many students miss: the pH scale is logarithmic. This means a solution with pH 6 has ten times more H⁺ ions than a solution with pH 7, and a hundred times more than pH 8.
For biological systems, this logarithmic nature means small pH changes can dramatically affect protein function. Most enzymes only function within narrow pH ranges, typically between 6-8, with some notable exceptions.
Real-World Connection: The $2.1 Billion Antacid Market
Your stomach produces hydrochloric acid (pH ~1.5) to digest food, but this same acid can cause problems if it escapes into your esophagus. The antacid industry exists entirely because of pH chemistry – these medications work by neutralizing excess stomach acid.
Buffers: The Body’s pH Control System
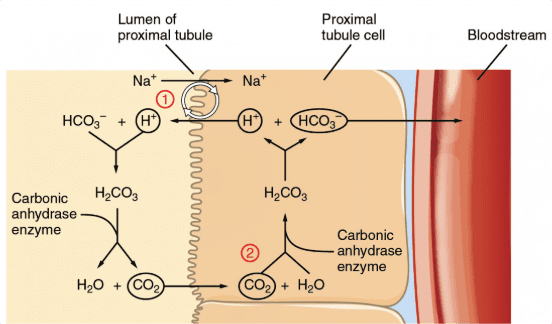
Living organisms use buffer systems to maintain stable pH levels. A buffer is a solution that resists changes in pH when acids or bases are added. The most important buffer in your blood is the bicarbonate buffer system (H₂CO₃/HCO₃⁻), which keeps your blood pH around 7.4.
Here’s how it works: If your blood becomes too acidic (from exercise producing lactic acid, for example), bicarbonate ions (HCO₃⁻) can bind with excess H⁺ ions. If your blood becomes too basic, carbonic acid (H₂CO₃) can release H⁺ ions. This system is so crucial that a blood pH change of just 0.3 units can be fatal.
Study Tip: Buffer Practice
When practicing buffer problems, always ask: “What happens when I add acid? What happens when I add base?” The buffer component with the opposite charge will respond.
Biological Macromolecules: The Building Blocks of Life
Now we’re getting to the heart of biochemistry – the four major types of biological macromolecules. These aren’t just random chemicals; they’re the molecular machines, structural materials, and information storage systems that make life possible.
Carbohydrates: More Than Just Energy
Most students think carbohydrates are just “sugar and starch for energy,” but that’s selling them short. Carbohydrates serve structural roles (cellulose in plant cell walls, chitin in insect exoskeletons), recognition roles (glycoproteins on cell surfaces), and energy storage roles (glycogen in animals, starch in plants).
Monosaccharides: The Simple Sugars
Glucose, fructose, and galactose are the most important monosaccharides. They all have the same molecular formula (C₆H₁₂O₆) but different structural arrangements. This is your first introduction to a crucial biological concept: structure determines function.
Glucose is the primary energy currency of cells. Its ring structure allows it to be easily modified – add a phosphate group and it can’t leave the cell, link multiple glucose molecules together and you get storage polymers or structural materials.
Disaccharides: Functional Partnerships
When two monosaccharides join via dehydration synthesis, they form disaccharides. Sucrose (table sugar) is glucose + fructose. Lactose (milk sugar) is glucose + galactose. Maltose is glucose + glucose.
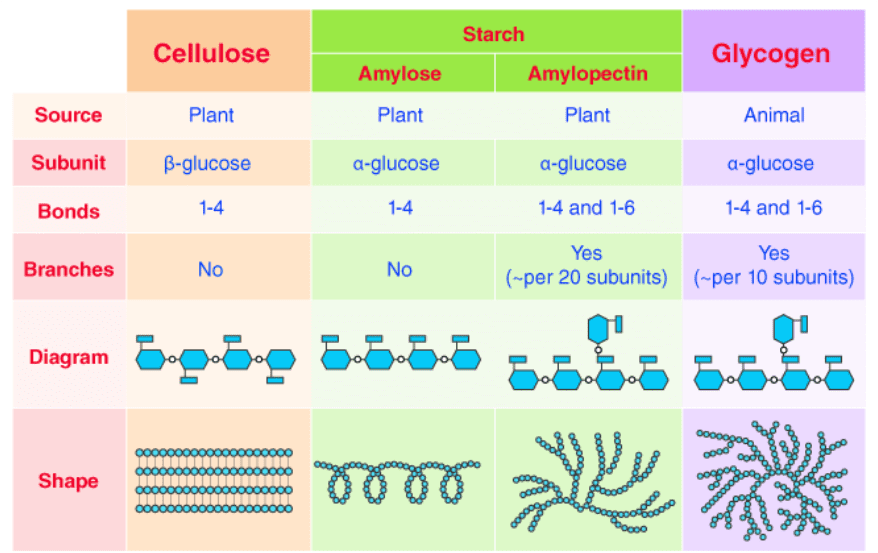
Polysaccharides: Polymers with Purpose
- Starch: Plant energy storage, easily digestible by humans
- Glycogen: Animal energy storage, highly branched for quick glucose release
- Cellulose: Plant structural support, β-1,4 linkages make it indigestible to most animals
- Chitin: Structural polymer in fungi and arthropods, contains nitrogen
Common Mistake Alert: Alpha vs. Beta Linkages
The difference between starch (α-1,4 linkages) and cellulose (β-1,4 linkages) is just the orientation of one hydroxyl group, but it completely changes the molecule’s properties. Humans can digest starch but not cellulose because our enzymes are specific for α-linkages.
Lipids: The Hydrophobic Helpers
Lipids are united not by structure but by their shared hydrophobic nature. This makes them perfect for energy storage (more calories per gram than carbohydrates), insulation, signaling, and most importantly, forming biological membranes.
Fatty Acids: The Building Blocks
Fatty acids are long hydrocarbon chains with a carboxyl group (-COOH) at one end. The length and saturation of these chains determine their properties. Saturated fatty acids (no double bonds) pack tightly and are solid at room temperature. Unsaturated fatty acids (one or more double bonds) have kinks that prevent tight packing, keeping them liquid.
Triglycerides: Energy Storage Specialists
Three fatty acids attached to a glycerol backbone create triglycerides – your body’s long-term energy storage molecules. They store more than twice the energy per gram compared to carbohydrates because they’re almost entirely hydrocarbon (which releases lots of energy when oxidized).
Phospholipids: Membrane Makers
Replace one fatty acid in a triglyceride with a phosphate group, and you get a phospholipid – a molecule with a hydrophilic head and hydrophobic tails. These spontaneously arrange into bilayers in water, creating the foundation of all biological membranes.
Steroids: Signaling and Structure
Built around a four-ring structure, steroids include cholesterol (membrane fluidity regulation), testosterone and estrogen (sex hormones), and cortisol (stress hormone). Despite their bad reputation in sports, steroids are essential biological molecules.
Study Tip: Lipid Functions Memory Device
Remember “SIMS” – Signaling, Insulation, Membranes, Storage. Every lipid you encounter will serve at least one of these functions.
Proteins: The Molecular Machines
If DNA is the blueprint of life, proteins are the construction workers, tools, and machinery. They’re involved in virtually every biological process, which makes understanding their structure and function crucial for AP Biology success.
Amino Acids: The Protein Alphabet
Twenty different amino acids combine in countless ways to create proteins. Each amino acid has the same basic structure – an amino group (NH₂), a carboxyl group (COOH), and a unique R group (side chain) attached to a central carbon.
The R groups determine each amino acid’s properties:
- Polar R groups: Can form hydrogen bonds (serine, threonine, tyrosine)
- Nonpolar R groups: Hydrophobic interactions (alanine, valine, leucine)
- Charged R groups: Ionic interactions (lysine +, aspartic acid -)
- Special cases: Cysteine (forms disulfide bonds), proline (creates kinks), glycine (small and flexible)
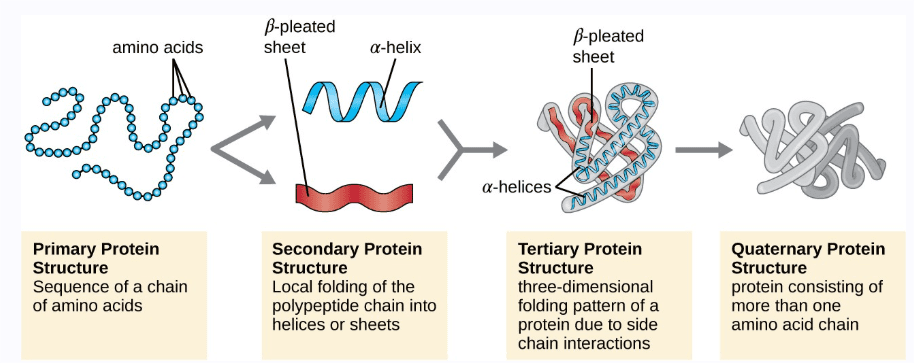
The Four Levels of Protein Structure
Primary Structure: The linear sequence of amino acids, determined by DNA. Change even one amino acid (like in sickle cell anemia), and you can change the entire protein’s function.
Secondary Structure: Local folding patterns stabilized by hydrogen bonds between backbone atoms. α-helices are springy and flexible; β-sheets are strong and rigid. Think of these as the basic architectural elements.
Tertiary Structure: The overall 3D shape of a single protein chain, stabilized by interactions between R groups. This is where the protein gets its specific function – the shape of the active site determines what the protein can do.
Quaternary Structure: Multiple protein chains working together. Hemoglobin has four subunits that cooperate to carry oxygen efficiently.
Protein Folding: Why Shape Matters
Proteins must fold into specific shapes to function. The primary structure determines how the protein will fold, but the process can go wrong. Misfolded proteins cause diseases like Alzheimer’s, Parkinson’s, and Creutzfeldt-Jakob disease.
Real-World Connection: The $15 Billion Protein Therapeutics Market
Modern medicine increasingly relies on protein-based drugs. Insulin for diabetes, growth hormone for growth disorders, and monoclonal antibodies for cancer treatment all work because scientists understand protein structure and function.
Nucleic Acids: Information Storage and Transfer
DNA and RNA are the information molecules of life. They store, transmit, and express genetic information using a surprisingly simple four-letter alphabet.
Nucleotide Structure
Every nucleotide has three parts: a phosphate group, a five-carbon sugar (ribose in RNA, deoxyribose in DNA), and a nitrogenous base (A, T, G, C in DNA; A, U, G, C in RNA).
DNA Structure and Function
DNA’s double helix structure, with complementary base pairing (A-T, G-C), allows it to store information reliably and replicate accurately. The sugar-phosphate backbone provides structural stability, while the bases store information through their sequence.
RNA’s Versatile Roles
RNA is single-stranded and more versatile than DNA. mRNA carries genetic information from nucleus to ribosomes. tRNA brings amino acids to the ribosome during protein synthesis. rRNA forms part of ribosome structure. Various other RNAs regulate gene expression.
Quick Check: Macromolecule Functions
Match each function to the correct macromolecule type:
- Long-term energy storage: –
- Genetic information storage: –
- Catalyzing biochemical reactions: –
- Forming cell membranes: –
- Quick energy for cellular processes: –
Enzymes: The Biological Catalysts
Enzymes are perhaps the most important proteins you’ll study in AP Biology. They make life possible by speeding up chemical reactions that would otherwise occur too slowly to sustain life. Understanding enzyme function is crucial because enzymes are involved in virtually every biological process.
How Enzymes Work: Lowering the Energy Barrier
Every chemical reaction requires activation energy – the energy needed to break existing bonds before new ones can form. Enzymes work by lowering this activation energy, making reactions occur much faster.
The enzyme-substrate model explains this process:
- Substrate binding: The substrate (reactant) binds to the enzyme’s active site
- Induced fit: Both enzyme and substrate change shape slightly to optimize binding
- Catalysis: The enzyme stabilizes the transition state, lowering activation energy
- Product release: Products form and leave the active site, freeing the enzyme
The Lock-and-Key vs. Induced Fit Models
The old lock-and-key model suggested rigid enzyme-substrate binding. The more accurate induced fit model recognizes that both molecules change shape during binding, optimizing the reaction.
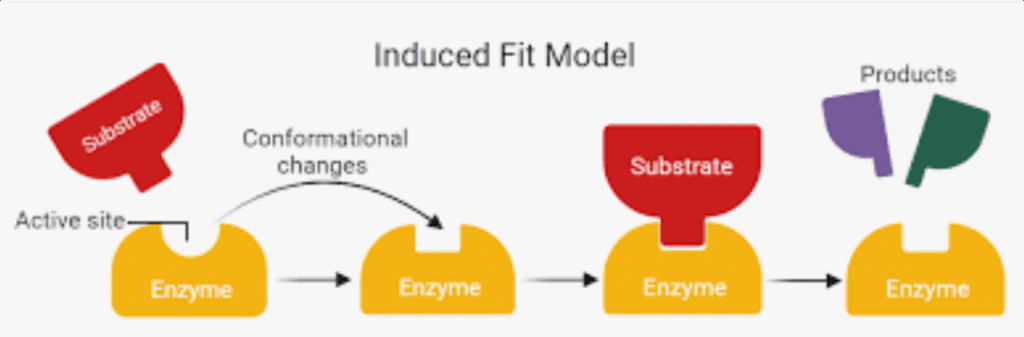
Factors Affecting Enzyme Activity
Understanding what affects enzyme function is crucial for AP exam success. These factors appear in countless exam questions and lab scenarios.
Temperature Effects
As temperature increases, molecular motion increases, leading to more enzyme-substrate collisions and faster reaction rates. However, excessive heat denatures enzymes by disrupting their tertiary structure. Most human enzymes work optimally around 37°C (body temperature).
pH Effects
Enzymes have optimal pH ranges where they maintain proper shape and charge distribution. Pepsin works best at pH 1.5 (stomach acid), while trypsin prefers pH 8 (small intestine). Outside optimal pH ranges, enzymes denature or lose activity.
Substrate Concentration
At low substrate concentrations, increasing substrate increases reaction rate proportionally. At high concentrations, enzymes become saturated – all active sites are occupied, and adding more substrate doesn’t increase the rate.
Enzyme Concentration
More enzymes mean more active sites available for catalysis, directly increasing reaction rate (assuming substrate isn’t limiting).
Competitive vs. Non-competitive Inhibition
- Competitive inhibitors compete with substrate for the active site. Increasing substrate concentration can overcome competitive inhibition.
- Non-competitive inhibitors bind elsewhere on the enzyme, changing its shape and reducing activity. Substrate concentration changes don’t affect non-competitive inhibition.
Study Tip: Enzyme Kinetics Graphs
Learn to interpret Michaelis-Menten curves. The curve approaches Vmax (maximum reaction rate) as substrate concentration increases. KM (Michaelis constant) is the substrate concentration at half-maximum velocity – lower KM means higher enzyme affinity for substrate.
Real-World Connection: The $7 Billion Enzyme Market
Industrial enzymes are used in everything from detergents (proteases break down protein stains) to biofuels (cellulases break down plant cell walls). Understanding enzyme chemistry opens doors to biotechnology careers.
Allosteric Regulation: Enzymes That Change Shape
Many enzymes can exist in active or inactive forms, controlled by regulatory molecules binding to sites other than the active site. This allosteric regulation allows cells to control metabolic pathways.
Positive regulation: Activators bind and increase enzyme activity
Negative regulation: Inhibitors bind and decrease enzyme activity
Feedback inhibition: The end product of a metabolic pathway inhibits the first enzyme, preventing overproduction
Chemical Bonds and Molecular Interactions in Biology
Understanding the types of chemical bonds and interactions is fundamental to grasping how biological molecules function and interact.
Covalent Bonds: The Strong Connections
Covalent bonds form when atoms share electrons. In biological molecules, these provide the strong backbone structure. The C-C and C-H bonds in organic molecules are covalent bonds that give these molecules stability.
Polar vs. Nonpolar Covalent Bonds
When atoms with different electronegativities share electrons, the sharing isn’t equal, creating polar covalent bonds. The O-H bonds in water are polar covalent, giving water its special properties.
Non-Covalent Interactions: The Weak Forces That Matter
While weaker than covalent bonds, non-covalent interactions are crucial for biological function because they can form and break quickly, allowing for dynamic processes.
Hydrogen Bonds
We’ve discussed these in the context of water, but they’re everywhere in biology. Hydrogen bonds:
- Hold DNA’s double helix together
- Stabilize protein secondary structure
- Allow enzymes to bind substrates specifically
- Enable specific base pairing in nucleic acids
Van der Waals Forces
Weak attractions between atoms in close proximity. These contribute to protein folding and the hydrophobic effect that drives membrane formation.
Ionic Interactions
Attractions between charged molecules. Important in protein structure (salt bridges) and in the function of many enzymes.
Common Mistake Alert: Bond Strength vs. Biological Importance
Students often think stronger bonds are always more important. In biology, weak interactions are often more important because they allow for the flexibility and specificity needed for biological processes.
Energy and Thermodynamics in Biological Systems
Living organisms must constantly input energy to maintain their highly organized state. Understanding energy flow is crucial for later units on metabolism and ecology.
The Laws of Thermodynamics in Biology
First Law: Energy cannot be created or destroyed, only transformed. In biological systems, light energy becomes chemical energy (photosynthesis), chemical energy becomes mechanical energy (muscle contraction), etc.
Second Law: Entropy (disorder) of the universe always increases. Living organisms maintain their organization by constantly using energy and increasing entropy in their surroundings.
Free Energy and Spontaneous Reactions
Gibbs free energy (G) determines whether reactions occur spontaneously. Reactions with negative ΔG are spontaneous; those with positive ΔG require energy input.
Cells couple energetically unfavorable reactions (positive ΔG) with favorable ones (negative ΔG) to drive necessary processes. ATP hydrolysis is often used to provide energy for unfavorable reactions.
ATP: The Universal Energy Currency
Adenosine triphosphate (ATP) stores energy in its phosphate bonds. When these bonds break (hydrolysis), energy is released for cellular work. The ATP-ADP cycle constantly recycles, with ATP being regenerated through cellular respiration.
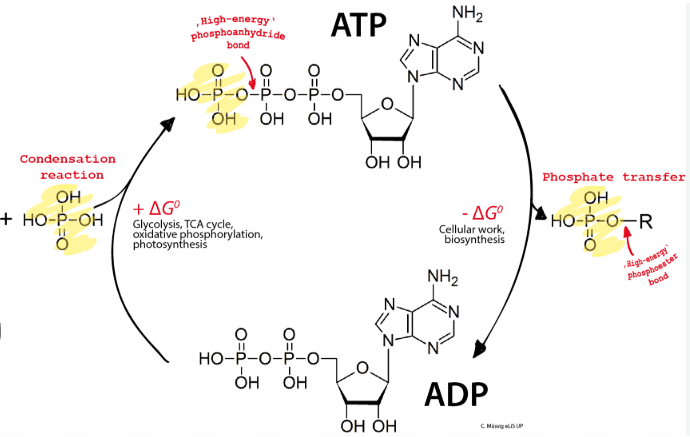
Practice Questions and Exam Strategies
Multiple Choice Practice Questions
Question 1: Which of the following best explains why ice floats on water?
A) Ice molecules move faster than liquid water molecules
B) Ice has a lower density than liquid water due to hydrogen bonding
C) Ice contains more hydrogen bonds than liquid water
D) Ice molecules are smaller than liquid water molecules
Answer: B. When water freezes, hydrogen bonds lock molecules into a rigid crystalline structure that’s less dense than liquid water.
Question 2: An enzyme’s active site is most directly related to its:
A) Primary structure only
B) Secondary structure only
C) Tertiary structure only
D) Quaternary structure only
Answer: C. The active site’s shape depends on how the entire protein chain folds (tertiary structure).
Question 3: Which of the following would most likely denature an enzyme?
A) Decreasing substrate concentration
B) Adding a competitive inhibitor
C) Increasing temperature to 80°C
D) Adding more enzyme molecules
Answer: C. High temperatures disrupt the weak interactions maintaining protein structure.
Free Response Practice
Sample FRQ: Enzymes are crucial for biological processes.
(a) Explain how enzyme structure relates to function, including the role of the active site.
(b) Describe how temperature affects enzyme activity and explain the molecular basis for these effects.
(c) A researcher discovers that compound X inhibits enzyme Y. Design an experiment to determine whether compound X is a competitive or non-competitive inhibitor.
Sample Answer Framework:
(a) Enzyme function depends on precise 3D structure, particularly the active site shape that determines substrate specificity through complementary binding…
(b) Moderate temperature increases molecular motion and reaction rates, but excessive heat denatures enzymes by disrupting weak interactions…
(c) Test varying substrate concentrations with and without compound X. If inhibition decreases with higher substrate concentration, it’s competitive…
Data Analysis Practice
Graph Interpretation: Given a graph showing reaction rate vs. substrate concentration for an enzyme at different temperatures, students should be able to:
- Identify optimal temperature
- Explain why the curve plateaus (enzyme saturation)
- Predict effects of temperature changes
- Calculate relative reaction rates
Common Exam Mistakes and How to Avoid Them
Mistake 1: Confusing correlation with causation
Just because two variables change together doesn’t mean one causes the other. Always look for mechanisms.
Mistake 2: Ignoring the logarithmic nature of pH
A change from pH 6 to pH 4 represents a 100-fold increase in H⁺ concentration, not just “2 units.”
Mistake 3: Oversimplifying enzyme inhibition
Competitive inhibition can be overcome by increasing substrate; non-competitive cannot. Know the difference.
Mistake 4: Memorizing without understanding
Don’t just memorize that enzymes are “biological catalysts.” Understand HOW they speed up reactions by lowering activation energy.
Real-World Applications and Current Research
Biotechnology Applications
Understanding the chemistry of life has led to revolutionary biotechnologies:
Protein Engineering: Scientists design new enzymes for industrial processes, creating more efficient catalysts for manufacturing everything from pharmaceuticals to biofuels.
Biomaterials: Understanding protein folding has led to engineered spider silk proteins that are stronger than steel and biodegradable plastics made from modified plant proteins.
Personalized Medicine: Knowledge of how genetic variations affect protein function allows doctors to prescribe medications based on individual enzyme variants.
Current Research Frontiers
Protein Folding Prediction: AI systems like AlphaFold can now predict protein structures from amino acid sequences, revolutionizing drug discovery and protein engineering.
Enzyme Evolution: Scientists are using directed evolution to create enzymes that can break down plastic waste, potentially solving environmental problems.
Chemical Biology: Researchers are creating artificial amino acids and modified nucleotides to expand the chemical capabilities of biological systems.
Study Tip: Connecting to Current Events
Follow science news related to biochemistry. Recent Nobel Prizes, FDA drug approvals, and environmental technologies often relate directly to Unit 1 concepts.
Mastering Unit 1: Your Study Strategy
Time Management for Unit 1
Week 1: Focus on water properties and basic chemistry concepts. These are foundational – everything else builds on them.
Week 2: Dive deep into macromolecule structure and function. Use molecular models or online visualizations to understand 3D structures.
Week 3: Master enzyme kinetics and regulation. Practice interpreting graphs and experimental data.
Week 4: Integration and practice. Work on connecting Unit 1 concepts to each other and to other AP Bio units.
Active Learning Strategies
Concept Mapping: Create visual connections between different Unit 1 topics. How do water’s properties relate to protein folding? How do enzymes depend on proper pH?
Teach Back Method: Explain concepts to classmates, family members, or even pets. If you can’t explain it simply, you don’t understand it well enough.
Practice Problems: Work through enzyme kinetics problems, pH calculations, and molecular structure identifications regularly.
Laboratory Connections: Connect Unit 1 concepts to any labs you perform. How does the enzyme lab demonstrate concepts from class? How do buffer labs show chemistry principles in action?
Self-Assessment Checklist
Before moving to Unit 2, make sure you can:
□ Explain why water’s properties make life possible
□ Describe the structure and function of all four macromolecule types
□ Interpret enzyme kinetics graphs and experimental data
□ Predict how environmental changes affect biological molecules
□ Connect molecular structure to biological function
□ Solve problems involving pH, buffers, and chemical bonds
□ Apply thermodynamic principles to biological processes
Integration with Other AP Biology Units
Unit 1 concepts appear throughout the entire AP Biology curriculum:
Unit 2 (Cell Structure): Membrane structure depends on phospholipid chemistry and protein function.
Unit 3 (Cellular Energetics): Enzyme function drives metabolic pathways in cellular respiration and photosynthesis.
Unit 4 (Cell Communication): Signaling molecules are often proteins or small molecules following chemical principles.
Units 5-6 (Heredity): DNA and RNA structure and function build directly on nucleic acid chemistry.
Units 7-8 (Evolution/Ecology): Molecular evolution and biochemical cycles require understanding of chemical principles.
Final Thoughts: From Molecules to Life
As you master Unit 1, remember that you’re not just learning isolated facts about chemistry – you’re discovering the fundamental principles that make you possible. Every breath you take, every thought you think, every movement you make depends on the elegant molecular choreography you’ve just learned about.
The beauty of biochemistry lies in its universality. The same chemical principles that govern enzyme function in your cells also work in bacteria, plants, and every living thing on Earth. When you understand how hydrogen bonds stabilize DNA or how protein folding determines function, you’re grasping concepts that unite all of biology.
Your Next Steps:
- Complete the self-assessment checklist above
- Practice with additional problems from your textbook
- Form study groups to discuss challenging concepts
- Connect Unit 1 to current events and research
- Prepare to see these concepts applied throughout the rest of AP Biology
Remember, mastering Unit 1 isn’t just about passing the AP exam (though it will definitely help with that). You’re building the foundation for understanding life itself at the molecular level. Whether you pursue medicine, research, biotechnology, or any other science field, these concepts will serve as your fundamental toolkit for understanding the living world.
The chemistry of life isn’t just academic content – it’s the operating manual for the most sophisticated machines in the universe: living organisms. Master these concepts, and you’ll have the keys to understanding how life works, from the smallest virus to the largest whale, from the simplest bacteria to the complexity of your own brain.
Now go forth and ace that AP Biology exam – you’ve got the molecular foundation to understand anything the test throws at you!
Key Takeaways Summary:
- Water’s unique properties enable all biological processes
- The four macromolecules each serve specific, crucial functions
- Enzyme structure determines function through specific active sites
- Weak molecular interactions drive most biological processes
- Understanding chemistry is essential for all other biology concepts
- Unit 1 concepts integrate throughout the entire AP Biology curriculum
Final Study Reminder: Focus on understanding mechanisms rather than just memorizing facts. When you understand WHY water has unique properties or HOW enzymes lower activation energy, you can tackle any question format the AP exam presents.
Also Read –


2 thoughts on “Mastering AP Biology Unit 1: Chemistry of Life – Your Complete Guide to Acing the Exam”