The Fascinating World of Coordination Compounds
Have you ever wondered why blood is red or why leaves turn from green to brilliant yellow and orange in autumn? The answer lies in one of chemistry’s most elegant and beautiful topics – coordination compounds. These remarkable structures are literally everywhere around you, from the hemoglobin carrying oxygen in your bloodstream to the chlorophyll capturing sunlight in plants, from the vitamin B12 in your supplements to the colorful gemstones adorning jewelry.
Imagine chemistry as a grand orchestra, where simple atoms and molecules are individual musicians. Coordination compounds are like the symphony that emerges when these musicians come together in perfect harmony, creating something far more beautiful and complex than any single instrument could produce alone. In these compounds, a central metal atom acts like a conductor, orchestrating the arrangement of surrounding molecules and ions (called ligands) to create structures with extraordinary properties and functions.
What makes coordination compounds truly special is their ability to bridge the gap between simple inorganic chemistry and the complex biochemical processes that sustain life itself. When you take a deep breath, coordination compounds are at work. When plants photosynthesize, coordination compounds are the key players. When you receive medical treatment involving certain drugs, coordination compounds might be saving your life.
This comprehensive guide will take you on an exciting journey through the world of coordination compounds, transforming what might seem like abstract chemical theory into a vivid, understandable, and applicable body of knowledge. Whether you’re preparing for your CBSE Class 12 board exams, building a foundation for competitive entrance tests, or simply curious about the molecular mechanisms that make life possible, this guide will serve as your trusted companion.
Learning Objectives
By the end of this comprehensive study guide, you will be able to:
- Understand and Apply Werner’s Coordination Theory: Explain the fundamental principles behind coordination compound formation, including primary and secondary valencies, and predict the structure and properties of various coordination complexes.
- Master Nomenclature and Classification: Confidently name coordination compounds using IUPAC rules and classify them based on various criteria including ligand types, coordination numbers, and geometric arrangements.
- Analyze Bonding Theories: Compare and contrast Valence Bond Theory (VBT) and Crystal Field Theory (CFT) to explain the formation, stability, magnetic properties, and color of coordination compounds.
- Predict and Explain Properties: Determine coordination numbers, oxidation states, magnetic behavior, and optical properties of coordination compounds using theoretical frameworks.
- Connect Theory to Applications: Relate coordination compound principles to real-world applications in biochemistry, medicine, industry, and environmental science.
- Excel in CBSE Board Examinations: Solve numerical problems, answer conceptual questions, and tackle case studies with confidence using proven strategies and comprehensive practice.
1: Foundation Concepts – Building Your Coordination Chemistry Vocabulary
Before diving into the intricate world of coordination compounds, let’s establish a solid foundation by understanding the essential terminology and basic concepts. Think of this section as learning the alphabet before writing poetry – these fundamental ideas will support everything else you learn.
What Exactly Are Coordination Compounds?
A coordination compound is like a molecular apartment complex where a central metal ion (the landlord) provides space for various molecules or ions (the tenants) to live together in a organized, stable arrangement. More scientifically, coordination compounds are complex substances formed when simple salts are mixed together, creating new entities with properties entirely different from their components.
Consider the formation of [Cu(NH₃)₄]SO₄, a beautiful deep blue compound. When you mix copper sulfate (CuSO₄) with ammonia (NH₃), something magical happens. The pale blue copper sulfate transforms into an intensely blue solution, indicating the formation of a coordination compound. This isn’t just a physical mixture – it’s a new chemical entity with unique properties.
![Formation of [Cu(NH₃)₄]SO₄ showing the central Cu²⁺ ion surrounded by four NH₃ molecules in a square planar arrangement](https://solvefyai.com/wp-content/uploads/2025/09/image-387.png)
Key Players in Coordination Chemistry
Central Metal Ion/Atom: This is the star of the show, typically a transition metal ion that has empty orbitals available to accept electron pairs. Think of it as a magnetic personality that attracts and organizes everything around it. Common examples include Cu²⁺, Fe³⁺, Co³⁺, Ni²⁺, and Zn²⁺.
Ligands: These are the supporting cast – molecules or ions that donate electron pairs to the central metal. They’re like loyal friends who stick close to the central metal, providing stability and determining the compound’s properties. Ligands can be:
- Monodentate: Having one binding site (like NH₃, H₂O, Cl⁻)
- Bidentate: Having two binding sites (like ethylenediamine, oxalate ion)
- Polydentate: Having multiple binding sites (like EDTA with six binding sites)
Coordination Sphere: This is the immediate family of the central metal – the metal atom plus all directly bonded ligands, usually written in square brackets like [Cu(NH₃)₄]²⁺.
Counter Ions: These are like distant relatives that balance the charge but don’t participate directly in bonding with the central metal.
Chemistry Check: Can you identify the central metal ion, ligands, and counter ions in K₄[Fe(CN)₆]? How many ligands are present, and what is their type?
The Driving Forces Behind Coordination
Why do coordination compounds form at all? The answer lies in thermodynamics and the quest for stability. When ligands approach a metal ion, several factors come into play:
- Electrostatic Attraction: Opposite charges attract, providing the initial driving force
- Orbital Overlap: Sharing of electrons creates covalent character in the bonds
- Stabilization: The resulting complex is often more stable than separate components
- Entropy Effects: Sometimes the formation increases overall disorder, making it favorable
Real-World Chemistry Callout:
The formation of coordination compounds isn’t just academic theory. Your body uses this principle constantly. The iron in hemoglobin forms coordination bonds with oxygen, allowing your blood to transport this vital gas throughout your body. Without coordination chemistry, complex life as we know it couldn’t exist!
2: Werner’s Coordination Theory – The Foundation of Modern Coordination Chemistry
Alfred Werner, often called the father of coordination chemistry, revolutionized our understanding of these compounds in the early 1900s. His theory was so groundbreaking that it earned him the Nobel Prize in Chemistry in 1913, making him the first inorganic chemist to receive this honor.
The Mystery That Started It All
Imagine you’re a 19th-century chemist trying to understand why CoCl₃·6NH₃ behaves so differently from simple salts. When you dissolve it in water and add silver nitrate, only some of the chlorine atoms precipitate as AgCl. This was the puzzle that led Werner to his revolutionary insights.
Werner’s Key Insights
Primary and Secondary Valencies: Werner proposed that metals exhibit two types of valencies:
- Primary Valency (Oxidation State): This is the charge on the metal ion, satisfied by counter ions. It’s like the metal’s official job title.
- Secondary Valency (Coordination Number): This represents the number of ligands directly bonded to the metal. It’s like the metal’s actual working relationships.
For [Co(NH₃)₆]Cl₃:
- Primary valency of Co = +3 (satisfied by 3 Cl⁻ ions)
- Secondary valency of Co = 6 (satisfied by 6 NH₃ molecules)
![Representation of [Co(NH₃)₆]Cl₃ showing primary and secondary valencies with different line styles](https://solvefyai.com/wp-content/uploads/2025/09/image-388.png)
Geometric Arrangements
Werner didn’t just stop at identifying bonding patterns; he proposed specific three-dimensional arrangements for different coordination numbers:
Coordination Number 2: Linear (rare)
Coordination Number 4: Tetrahedral or Square planar
Coordination Number 6: Octahedral (most common)
This was revolutionary because it introduced the concept of stereochemistry to inorganic compounds, showing that they could exhibit the same spatial complexity as organic molecules.
Process Analysis: Applying Werner’s Theory
Let’s work through a systematic approach to analyzing coordination compounds using Werner’s theory:
- Identify the central metal ion: Look for the transition metal
- Determine the oxidation state: Use charge balance
- Count the ligands: Identify directly bonded species
- Predict geometry: Use coordination number and electronic factors
- Write the formula: Place coordination sphere in brackets
Example: K₃[Fe(CN)₆]
- Central metal: Fe
- Oxidation state: +3 (since 3K⁺ and overall neutral compound)
- Ligands: 6 CN⁻ ions
- Geometry: Octahedral (coordination number 6)
- Formula: K₃[Fe(CN)₆] with [Fe(CN)₆]³⁻ as coordination sphere
Evidence Supporting Werner’s Theory
Werner’s theory wasn’t just elegant speculation – he provided experimental evidence:
Conductance Studies: Different compounds showed different conductivity patterns, supporting his ideas about which ions were free in solution versus bound in the coordination sphere.
Isomerism: Werner predicted and synthesized geometric and optical isomers, proving that coordination compounds have definite three-dimensional structures.
Chemical Reactivity: The theory explained why some chloride ions in coordination compounds precipitate with AgNO₃ while others don’t.
Common Error Alert:
Students often confuse coordination number with oxidation state. Remember: coordination number counts ligands (secondary valency), while oxidation state indicates the charge on the metal (primary valency). They’re related but distinct concepts!
3: Ligands – The Supporting Cast That Steals the Show
If the central metal ion is the star of coordination chemistry, then ligands are the versatile supporting actors that can completely transform the performance. Understanding ligands is crucial because they determine the properties, stability, and applications of coordination compounds.
Classification of Ligands by Binding Sites
Monodentate Ligands: These are the solo performers, binding through a single atom. Think of them as having one hand to shake with the metal:
- Neutral molecules: H₂O (aqua), NH₃ (ammine), CO (carbonyl), NO (nitrosyl)
- Anions: Cl⁻ (chloro), Br⁻ (bromo), OH⁻ (hydroxo), CN⁻ (cyano)
Bidentate Ligands: These are like dancers performing a pas de deux, using two binding sites to create a ring with the metal:
- Ethylenediamine (en): H₂N-CH₂-CH₂-NH₂
- Oxalate ion (ox): ⁻OOC-COO⁻
- Bipyridine (bipy): Two pyridine rings connected
Polydentate Ligands: These are the ensemble performers, capable of binding through multiple sites. The most famous is EDTA (ethylenediaminetetraacetate), which can bind through six different atoms, wrapping around the metal like a molecular octopus.
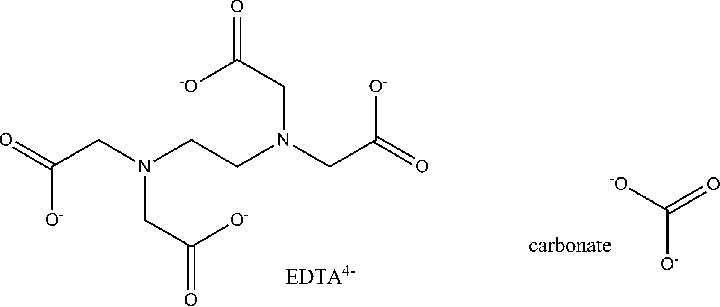
The Chelate Effect – Nature’s Molecular Embrace
When a polydentate ligand binds to a metal ion, it forms a chelate (from the Greek word “chela,” meaning claw). This creates a particularly stable arrangement because:
- Entropy Advantage: Replacing multiple monodentate ligands with one polydentate ligand increases disorder
- Reduced Dissociation: For the complex to fall apart, multiple bonds must break simultaneously
- Ring Stability: The resulting ring structures are inherently stable
Consider the replacement of six water molecules around a metal ion with three ethylenediamine molecules:
[M(H₂O)₆]²⁺ + 3en → [M(en)₃]²⁺ + 6H₂O
This reaction is highly favorable because we go from 4 particles to 7 particles, increasing entropy.
Ligand Field Strength and the Spectrochemical Series
Not all ligands are created equal when it comes to their interaction with metal d-orbitals. The spectrochemical series ranks ligands by their field strength:
Weak Field → Strong Field
I⁻ < Br⁻ < Cl⁻ < F⁻ < OH⁻ < H₂O < NH₃ < en < NO₂⁻ < CN⁻ < CO
This series is incredibly important because it determines:
- Color of the complex
- Magnetic properties
- Stability
- Reactivity
Real-World Chemistry Callout:
The spectrochemical series explains why [Fe(H₂O)₆]³⁺ is pale yellow while [Fe(CN)₆]³⁻ is deep red. The stronger field created by CN⁻ ligands causes a larger energy gap between d-orbitals, resulting in absorption of different wavelengths of light.
Special Types of Ligands
Ambidentate Ligands: These are the shape-shifters of coordination chemistry, capable of binding through different atoms. Examples include:
- NO₂⁻: Can bind through nitrogen (nitro) or oxygen (nitrito)
- SCN⁻: Can bind through sulfur (thiocyanato) or nitrogen (isothiocyanato)
Bridging Ligands: These ligands can simultaneously coordinate to two different metal centers, creating polynuclear complexes. Common bridging ligands include OH⁻, O²⁻, and Cl⁻.
π-Acceptor Ligands: Ligands like CO, NO, and CN⁻ not only donate electron density to the metal but can also accept electron density back into their empty π* orbitals. This creates exceptionally strong metal-ligand bonds.
Chemistry Check: A complex contains a metal ion with coordination number 6. If it contains 2 bidentate ligands, how many monodentate ligands are also present? What would be the geometry?
4: Nomenclature – Speaking the Language of Coordination Chemistry
Learning to name coordination compounds is like learning a new language – it might seem daunting at first, but once you understand the rules, it becomes a powerful tool for communication. The IUPAC (International Union of Pure and Applied Chemistry) has established systematic rules that allow chemists worldwide to understand exactly which compound is being discussed.
The Art and Science of Naming
Think of naming coordination compounds like describing a house address – you need to be specific enough that anyone can find exactly the right place. The name must convey the central metal, its oxidation state, the types and numbers of ligands, and sometimes even the geometric arrangement.
IUPAC Rules for Naming Coordination Compounds
Rule 1: Order of Components
In the name, ligands are listed alphabetically before the central metal atom. In the formula, the coordination sphere (metal + ligands) is enclosed in square brackets.
Rule 2: Ligand Naming
- Neutral ligands: Usually keep their common names (aqua for H₂O, ammine for NH₃)
- Anionic ligands: End in “-o” (chloro for Cl⁻, cyano for CN⁻, oxalato for C₂O₄²⁻)
- Cationic ligands: End preserve original name but add “-ium” if needed
Rule 3: Numerical Prefixes
Use Greek prefixes to indicate the number of each type of ligand:
- mono (1), di (2), tri (3), tetra (4), penta (5), hexa (6)
- For complex ligand names, use bis (2), tris (3), tetrakis (4), pentakis (5), hexakis (6)
Rule 4: Central Metal Naming
- In cationic/neutral complexes: Use the element name
- In anionic complexes: Use the Latin name ending in “-ate”
- Always include oxidation state in Roman numerals in parentheses
Rule 5: Alphabetical Order
List ligands alphabetically, ignoring numerical prefixes
Process Analysis: Step-by-Step Naming
Let’s work through naming [Co(NH₃)₄Cl₂]Cl:
Step 1: Identify components
- Central metal: Co
- Ligands: 4 NH₃, 2 Cl⁻ (coordinated), 1 Cl⁻ (counter ion)
Step 2: Determine oxidation state
- Overall charge = +1 (from counter ion)
- Ligand charges: 4(0) + 2(-1) = -2
- Metal oxidation state: +1 – (-2) = +3
Step 3: Name ligands alphabetically
- ammine (NH₃) comes before chloro (Cl⁻)
- tetraamminedichloro
Step 4: Name the metal
- Cationic complex, so use “cobalt(III)”
Step 5: Add counter ion
- “chloride”
Final name: tetraamminedichlorocobalt(III) chloride
Common Ligand Names You Must Know
Neutral Ligands:
- H₂O → aqua
- NH₃ → ammine
- CO → carbonyl
- NO → nitrosyl
- en (ethylenediamine) → ethylenediamine
Anionic Ligands:
- F⁻ → fluoro
- Cl⁻ → chloro
- Br⁻ → bromo
- I⁻ → iodo
- OH⁻ → hydroxo
- CN⁻ → cyano
- NO₂⁻ → nitro
- SO₄²⁻ → sulfato
- C₂O₄²⁻ → oxalato
Special Cases and Complex Situations
Bridging Ligands: When ligands connect two metal centers, use “μ” (mu) prefix:
[(NH₃)₅Cr-OH-Cr(NH₃)₅]⁵⁺ → μ-hydroxobis[pentaamminechromium(III)]
Isomers: Sometimes geometric or optical isomers require additional descriptors:
- cis-[PtCl₂(NH₃)₂] → cis-diamminedichloroplatinum(II)
- trans-[PtCl₂(NH₃)₂] → trans-diamminedichloroplatinum(II)
Coordination Compounds as Ligands: When one coordination compound acts as a ligand to another metal, name the inner complex first.
Chemistry Check: Name the following compounds: (a) K₃[Fe(CN)₆], (b) [Cu(H₂O)₄]SO₄, (c) [Co(en)₃]Cl₃. Then write the formula for hexaamminecobalt(III) sulfate.
Common Error Alert:
Students often forget that “ammine” (NH₃ ligand) is spelled differently from “amine” (organic nitrogen compounds). Also, remember that in anionic complexes, the metal name changes – iron becomes “ferrate,” chromium becomes “chromate,” etc.
5: Bonding Theories – Understanding the Molecular Architecture
Understanding how atoms bond in coordination compounds is like being an architect who needs to know not just what a building looks like, but why it stands up and how all the pieces fit together. Two major theories help us understand coordination bonding: Valence Bond Theory (VBT) and Crystal Field Theory (CFT). Each offers unique insights into the electronic structure and properties of these fascinating compounds.
Valence Bond Theory (VBT) – The Orbital Overlap Perspective
Imagine bonding as a handshake between atoms – VBT focuses on how atomic orbitals from the metal and ligands overlap to form new, shared electron pair bonds. This theory was developed by Linus Pauling and provides an intuitive way to understand coordination compound formation.
Key Concepts of VBT
Hybridization: The central metal atom’s orbitals mix and reshape to accommodate incoming ligands. Think of this like a musical ensemble where individual instruments blend their sounds to create harmony.
Common Hybridization Patterns:
- sp³: Tetrahedral geometry (4 ligands)
- dsp²: Square planar geometry (4 ligands)
- sp³d²: Octahedral geometry (6 ligands)
Inner vs. Outer Orbital Complexes: This classification depends on whether d-orbitals from the inner shell participate in hybridization:
- Inner orbital complexes: Use (n-1)d orbitals, typically with strong field ligands
- Outer orbital complexes: Use nd orbitals, typically with weak field ligands
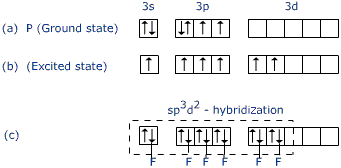
Predicting Properties Using VBT
Magnetic Behavior:
- Diamagnetic: All electrons paired (attracted to magnetic field)
- Paramagnetic: Unpaired electrons present (repelled by magnetic field)
Example Analysis: [Co(NH₃)₆]³⁺
- Co³⁺ configuration: [Ar] 3d⁶
- NH₃ is a strong field ligand, causing electron pairing
- Hybridization: d²sp³ (inner orbital)
- All electrons paired → diamagnetic
Stability: Inner orbital complexes are generally more stable due to:
- Better orbital overlap
- Stronger metal-ligand bonds
- More effective electron pairing
Crystal Field Theory (CFT) – The Electrostatic Perspective
While VBT focuses on orbital overlap, CFT takes a completely different approach, treating ligands as point charges that create an electrostatic field around the central metal ion. This theory, developed by Hans Bethe and John Hasbrouck van Vleck, excels at explaining color and magnetic properties.
The Core Idea of CFT
In a free metal ion, all five d-orbitals have the same energy. But when ligands approach, they create an electric field that affects different d-orbitals differently, causing them to split into groups with different energies.
Octahedral Crystal Field Splitting:
The five d-orbitals split into two groups:
- t₂g orbitals: dxy, dxz, dyz (lower energy, point between ligands)
- eg orbitals: dx²-y², dz² (higher energy, point toward ligands)
The energy difference between these groups is called the crystal field splitting energy (Δ₀).
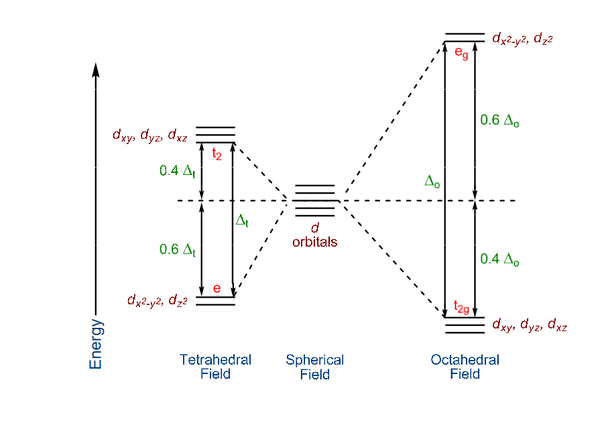
High Spin vs. Low Spin Complexes
The relative size of the crystal field splitting energy (Δ₀) compared to the electron pairing energy determines how electrons fill the d-orbitals:
High Spin (Weak Field):
- Δ₀ < Pairing Energy
- Electrons occupy all orbitals singly before pairing
- Maximum number of unpaired electrons
Low Spin (Strong Field):
- Δ₀ > Pairing Energy
- Electrons pair in lower orbitals before occupying higher ones
- Minimum number of unpaired electrons
Example: Fe³⁺ (d⁵) complexes
- [Fe(H₂O)₆]³⁺: High spin, 5 unpaired electrons
- [Fe(CN)₆]³⁻: Low spin, 1 unpaired electron
Tetrahedral Crystal Field Splitting
In tetrahedral complexes, the d-orbital splitting pattern is inverted:
- e orbitals: dz², dx²-y² (lower energy)
- t₂ orbitals: dxy, dxz, dyz (higher energy)
The splitting energy (Δt) is smaller than octahedral splitting, so tetrahedral complexes are almost always high spin.
Process Analysis: Determining Magnetic Properties Using CFT
Step 1: Identify the metal ion and its d-electron configuration
Step 2: Determine the geometry (octahedral, tetrahedral, square planar)
Step 3: Classify ligands as strong field or weak field
Step 4: Apply appropriate crystal field splitting
Step 5: Fill electrons according to high spin/low spin rules
Step 6: Count unpaired electrons to determine magnetic behavior
Color in Coordination Compounds
CFT beautifully explains why coordination compounds are often colored. When white light hits a complex, d-electrons can absorb specific wavelengths to jump from lower to higher energy orbitals. The color we see is the complement of the absorbed light.
The relationship: E = hν = hc/λ = Δ
For [Ti(H₂O)₆]³⁺:
- Ti³⁺ has d¹ configuration
- Absorbs green light (around 500 nm)
- Appears purple (complementary color)
Real-World Chemistry Callout:
The colors of gemstones often result from crystal field effects in coordination compounds. Ruby’s red color comes from Cr³⁺ ions in an octahedral field created by oxide ions in the aluminum oxide crystal structure. Sapphire’s blue color results from charge transfer between Fe²⁺ and Ti⁴⁺ ions.
Chemistry Check: For [Mn(H₂O)₆]²⁺ and [Mn(CN)₆]⁴⁻, predict which will be high spin and which will be low spin. How many unpaired electrons does each have?
6: Isomerism in Coordination Compounds – The Art of Molecular Arrangements
Isomerism in coordination compounds is like having multiple ways to arrange the same furniture in a room – you have the same pieces, but different arrangements create entirely different looks and functionalities. This phenomenon adds incredible diversity to coordination chemistry and has profound implications for biological activity, catalysis, and material properties.
Understanding Isomerism
Isomers are compounds with the same molecular formula but different arrangements of atoms. In coordination compounds, this concept becomes particularly rich because we can have different arrangements both within the coordination sphere and in the overall compound structure.
Structural Isomerism – Different Connectivity
Linkage Isomerism: This occurs when an ambidentate ligand can bind through different atoms. It’s like having a key that can open a lock from either end.
Classic Example: [Co(NH₃)₅(NO₂)]Cl₂ vs [Co(NH₃)₅(ONO)]Cl₂
- First isomer: NO₂⁻ binds through nitrogen (nitro linkage)
- Second isomer: NO₂⁻ binds through oxygen (nitrito linkage)
- Different colors and properties despite identical formulas
Coordination Isomerism: This happens when both cation and anion are complex ions, and ligands can be distributed differently between them.
Example: [Co(NH₃)₆][Cr(CN)₆] vs [Cr(NH₃)₆][Co(CN)₆]
- Same overall composition, different metal-ligand assignments
Ionization Isomerism: This occurs when ligands and counter ions can exchange positions.
Example: [Co(NH₃)₅Br]SO₄ vs [Co(NH₃)₅SO₄]Br
- First gives Br⁻ with AgNO₃ test
- Second gives SO₄²⁻ with BaCl₂ test
Hydrate Isomerism: This involves different arrangements of water molecules as ligands versus molecules of crystallization.
Example: [Cr(H₂O)₆]Cl₃ (violet) vs [Cr(H₂O)₄Cl₂]Cl·2H₂O (green)
Stereoisomerism – Same Connectivity, Different Spatial Arrangements
Geometric Isomerism: This is like having different seating arrangements at a dinner table – the same people are present, but their relative positions create different social dynamics.
In Square Planar Complexes (MA₂B₂):
- Cis isomer: Similar ligands adjacent (90° angle)
- Trans isomer: Similar ligands opposite (180° angle)
Famous Example: Cisplatin vs Transplatin
- cis-[PtCl₂(NH₃)₂]: Highly effective anticancer drug
- trans-[PtCl₂(NH₃)₂]: Biologically inactive
- Same formula, different geometry, completely different biological activity!
![Structures of cis and trans isomers of [PtCl₂(NH₃)₂] showing different spatial arrangements](https://solvefyai.com/wp-content/uploads/2025/09/image-392.png)
In Octahedral Complexes:
The possibilities become more complex with six coordination positions:
For MA₄B₂ complexes:
- Cis: Two B ligands adjacent (90° angle)
- Trans: Two B ligands opposite (180° angle)
For MA₃B₃ complexes:
- Fac (facial): Three identical ligands occupy one triangular face
- Mer (meridional): Three identical ligands span a meridian of the octahedron
Optical Isomerism – The Mirror Image Phenomenon
Optical isomerism occurs when a molecule and its mirror image cannot be superimposed, much like your left and right hands. These isomers, called enantiomers, have identical physical and chemical properties except for their interaction with polarized light and other chiral molecules.
Common Sources of Optical Activity:
- Chelate complexes: [Co(en)₃]³⁺ exists as Δ and Λ enantiomers
- Octahedral complexes with mixed ligands: [Co(NH₃)₄Cl₂]⁺
- Complexes with chiral ligands
![Optical isomers of [Co(en)₃]³⁺ showing Δ (delta) and Λ (lambda) forms as non-superimposable mirror images](https://solvefyai.com/wp-content/uploads/2025/09/image-393.png)
Biological Significance: Optical isomerism is crucial in biochemistry because enzymes and receptors are chiral. One enantiomer might be therapeutic while its mirror image could be toxic.
Historical Context:
Alfred Werner proved the octahedral structure of coordination compounds by resolving [Co(en)₂Cl₂]⁺ into optical isomers. This was groundbreaking because it showed that inorganic compounds could exhibit the same stereochemical complexity as organic molecules.
Process Analysis: Identifying Isomerism Types
Step 1: Examine the molecular formula – same formula suggests isomerism
Step 2: Check connectivity – different bonding patterns indicate structural isomerism
Step 3: If connectivity is same, look for geometric differences
Step 4: Examine symmetry – absence of symmetry elements suggests optical activity
Step 5: Consider biological or chemical property differences
Factors Affecting Isomer Stability
Electronic Effects: Different isomers may have different electronic stability due to:
- Crystal field stabilization energies
- π-bonding capabilities
- Electronic repulsion between ligands
Steric Effects: Bulky ligands prefer positions that minimize crowding:
- Trans positions in square planar complexes
- Facial arrangements might be sterically hindered
Thermodynamic vs Kinetic Control:
- Thermodynamic products are most stable
- Kinetic products form fastest
- Interconversion rates determine which isomer predominates
Chemistry Check: How many geometrical isomers are possible for [Ma₂b₂c₂] in octahedral geometry? Draw the structures and identify which ones might show optical isomerism.
Common Error Alert:
Students often confuse geometric and optical isomerism. Remember: geometric isomers have different physical arrangements of the same ligands (like cis/trans), while optical isomers are non-superimposable mirror images. A compound can show both types of isomerism simultaneously!
7: Applications and Importance – Coordination Chemistry in Action
The true beauty of coordination chemistry lies not just in its elegant theory, but in its incredible practical importance. From the moment you wake up until you go to sleep, coordination compounds are working behind the scenes to make your life possible, healthier, and more colorful. Let’s explore how these remarkable structures impact everything from the blood in your veins to the technology in your smartphone.
Biological Systems – The Chemistry of Life
Hemoglobin and Oxygen Transport: Perhaps the most vital coordination compound in your body is hemoglobin, where an iron(II) ion sits at the center of a porphyrin ring (a large, flat, nitrogen-containing ligand). This [Fe(porphyrin)] unit can reversibly bind oxygen, allowing your blood to carry O₂ from your lungs to every cell in your body.
The genius of this system lies in its tunability:
- In your lungs (high O₂, high pH): Hemoglobin has high oxygen affinity
- In your tissues (low O₂, low pH): Hemoglobin releases oxygen readily
- Carbon monoxide is dangerous because it binds 200 times more strongly than O₂
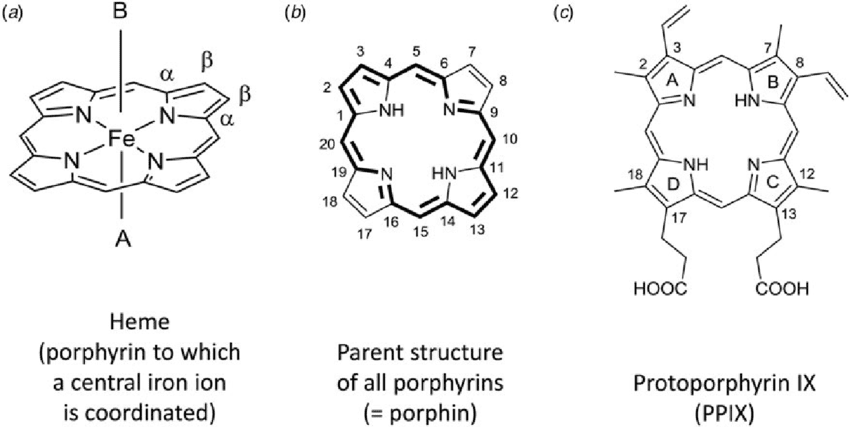
Chlorophyll and Photosynthesis: Life on Earth depends on plants’ ability to capture sunlight, and this process relies on chlorophyll – a magnesium coordination compound. The [Mg(porphyrin)] structure absorbs red and blue light while reflecting green, giving plants their characteristic color and enabling them to convert light energy into chemical energy.
Vitamin B₁₂: This essential vitamin contains a cobalt coordination compound with one of the most complex structures in biochemistry. The cobalt center can form organometallic bonds, making it crucial for several enzymatic reactions including DNA synthesis.
Enzymes: Many enzymes contain metal coordination centers:
- Carbonic anhydrase: Zinc center helps regulate blood pH
- Superoxide dismutase: Copper and zinc centers protect against oxidative damage
- Cytochrome oxidase: Copper and iron centers enable cellular respiration
Medical Applications – Healing Through Coordination
Anticancer Drugs: Cisplatin, [PtCl₂(NH₃)₂], revolutionized cancer treatment. It works by:
- Entering cancer cells
- Losing chloride ligands to become reactive
- Binding to DNA and preventing replication
- Causing cancer cell death
The success of cisplatin led to development of other platinum-based drugs like carboplatin and oxaliplatin, each designed to overcome specific limitations.
MRI Contrast Agents: Gadolinium coordination compounds like [Gd(DTPA)]²⁻ are used in MRI scans. The paramagnetic Gd³⁺ ion affects water relaxation times, creating contrast in images that helps doctors diagnose diseases.
Chelation Therapy: When someone has heavy metal poisoning, coordination compounds can save their life:
- EDTA: Removes lead, mercury, and other toxic metals
- Penicillamine: Treats Wilson’s disease (copper accumulation)
- Deferoxamine: Removes excess iron in iron overload conditions
Radioactive Imaging: Technetium-99m complexes are used in nuclear medicine for imaging hearts, bones, and other organs. The coordination environment affects where the complex localizes in the body.
Industrial Applications – Chemistry at Work
Catalysis: Coordination compounds are master catalysts, speeding up reactions that are essential for industrial processes:
Hydrogenation: Wilkinson’s catalyst, [RhCl(PPh₃)₃], adds hydrogen to alkenes under mild conditions, crucial for producing pharmaceuticals and fine chemicals.
Polymerization: Ziegler-Natta catalysts (titanium and aluminum coordination compounds) produce polyethylene and polypropylene, two of the most important plastics in the world.
Hydroformylation: Cobalt and rhodium complexes add CO and H₂ to alkenes, producing aldehydes used in plasticizers, detergents, and pharmaceuticals.
Homogeneous vs Heterogeneous Catalysis:
- Homogeneous: Catalyst in same phase as reactants, easier to study mechanism
- Heterogeneous: Catalyst on solid support, easier to separate and reuse
Analytical Chemistry – Detection and Measurement
Colorimetric Analysis: Many coordination compounds are intensely colored, making them perfect for quantitative analysis:
- Iron determination: Forms red complex with 1,10-phenanthroline
- Nickel analysis: Forms pink complex with dimethylglyoxime
- Copper detection: Forms blue complex with ammonia
Indicators: Coordination compounds serve as indicators in various types of titrations:
- EDTA titrations: Eriochrome Black T changes color when metal ions are complexed
- Redox titrations: Ferroin indicator changes from red to blue
Chromatography: Metal coordination affects how compounds move through chromatographic systems, enabling separation of complex mixtures.
Advanced Materials – Technology Applications
Magnetic Materials: Coordination compounds with unpaired electrons create materials with unique magnetic properties:
- Prussian Blue: Fe₄[Fe(CN)₆]₃, one of the first synthetic pigments, shows interesting magnetic behavior
- Single-molecule magnets: Individual coordination compounds that act as tiny magnets
Electronic Materials:
- Organic Light-Emitting Diodes (OLEDs): Iridium and platinum coordination compounds provide efficient light emission in different colors
- Solar cells: Ruthenium coordination compounds in dye-sensitized solar cells
Smart Materials: Coordination compounds that respond to external stimuli:
- Thermochromic materials: Change color with temperature
- pH sensors: Change properties with acidity
Environmental Applications – Protecting Our Planet
Water Treatment: Coordination compounds help remove pollutants:
- Heavy metal removal: Chelating agents bind toxic metals
- Photocatalytic degradation: Titanium dioxide coordination compounds break down organic pollutants
Green Chemistry: Coordination catalysts enable more efficient, environmentally friendly processes:
- Lower reaction temperatures: Reducing energy consumption
- Higher selectivity: Reducing waste production
- Renewable feedstocks: Converting biomass to useful chemicals
Real-World Chemistry Callout:
The next time you take a photograph, remember that the process likely involves silver coordination compounds in the photographic emulsion. When you drive a car with a catalytic converter, platinum group metal coordination compounds are cleaning your exhaust. When you see the brilliant colors in fireworks, coordination compounds are creating those spectacular displays.
Chemistry Check: Explain why carbon monoxide is so dangerous in terms of coordination chemistry. How does this relate to the treatment of CO poisoning with oxygen therapy?
8: Problem-Solving Strategies and Calculations
Mastering coordination chemistry requires more than just understanding concepts – you need to develop problem-solving skills that allow you to tackle numerical problems, predict properties, and analyze complex scenarios. Think of this section as your toolkit for becoming a coordination chemistry detective, equipped with systematic approaches to solve any challenge you encounter.
Systematic Approach to Coordination Compound Problems
The MORSE Method (Metal, Oxidation state, Rules, Structure, Electrons):
Metal: Identify the central metal atom/ion
Oxidation state: Determine the oxidation state of the metal
Rules: Apply relevant theories (VBT, CFT) and rules
Structure: Predict geometry and possible isomers
Electrons: Analyze electronic configuration and magnetic properties
Determining Oxidation States
This is often the first and most crucial step in solving coordination problems. Think of it as finding the “electrical balance” of the compound.
Method 1: Charge Balance
The sum of all charges must equal the overall charge of the compound.
Example: Determine the oxidation state of Cr in K₃[Cr(C₂O₄)₃]
- Overall charge = 0 (neutral compound)
- K⁺ contribution: 3 × (+1) = +3
- C₂O₄²⁻ contribution: 3 × (-2) = -6
- For charge balance: +3 + x + (-6) = 0
- Therefore: x = +3 (Cr is in +3 oxidation state)
Method 2: Known Ligand Charges
Memorize common ligand charges and use them systematically.
Common Ligand Charges:
- Neutral ligands: NH₃, H₂O, en, CO (charge = 0)
- Monovalent anions: Cl⁻, Br⁻, I⁻, CN⁻, OH⁻ (charge = -1)
- Divalent anions: SO₄²⁻, C₂O₄²⁻, CO₃²⁻ (charge = -2)
Process Analysis: Complete Problem Solution
Problem: For [Co(NH₃)₄Cl₂]Cl, determine: (a) oxidation state of Co, (b) coordination number, (c) possible isomers, (d) magnetic behavior.
Step 1 – Metal Identification: Co (cobalt)
Step 2 – Oxidation State:
- Overall charge = +1 (one Cl⁻ counter ion)
- NH₃ ligands: 4 × 0 = 0
- Cl⁻ ligands: 2 × (-1) = -2
- Charge balance: x + 0 + (-2) = +1
- Co oxidation state = +3
Step 3 – Coordination Number: 6 (4 NH₃ + 2 Cl⁻)
Step 4 – Geometry: Octahedral (coordination number 6)
Step 5 – Possible Isomers:
- Cis isomer: Two Cl⁻ ligands adjacent
- Trans isomer: Two Cl⁻ ligands opposite
Step 6 – Electronic Configuration:
- Co³⁺: [Ar] 3d⁶
- NH₃ is strong field ligand → low spin
- Electron arrangement in octahedral field: t₂g⁶ eg⁰
- All electrons paired → diamagnetic
Calculating Magnetic Moments
The magnetic moment (μ) provides information about the number of unpaired electrons:
Formula: μ = √[n(n+2)] Bohr magnetons
where n = number of unpaired electrons
Magnetic Behavior Classification:
- Diamagnetic: μ = 0 (all electrons paired)
- Paramagnetic: μ > 0 (unpaired electrons present)
Example Calculation: [Mn(H₂O)₆]²⁺
- Mn²⁺: [Ar] 3d⁵
- H₂O is weak field → high spin
- Electron configuration: t₂g³ eg²
- Number of unpaired electrons = 5
- μ = √[5(5+2)] = √35 = 5.92 BM
Crystal Field Stabilization Energy (CFSE) Calculations
CFSE quantifies the stabilization energy gained when d-electrons occupy lower energy orbitals in a crystal field.
For Octahedral Complexes:
- Each electron in t₂g orbital: -0.4Δ₀ stabilization
- Each electron in eg orbital: +0.6Δ₀ destabilization
- Pairing energy (P) must be considered for low spin complexes
Example: Calculate CFSE for [Fe(CN)₆]³⁻ (low spin)
- Fe³⁺: d⁵ configuration
- Low spin arrangement: t₂g⁵ eg⁰
- CFSE = 5(-0.4Δ₀) + 2P = -2.0Δ₀ + 2P
Stability Constants and Equilibrium
Coordination compounds exist in equilibrium with their components. The stability constant (formation constant) quantifies this equilibrium.
For stepwise formation:
M + L ⇌ ML K₁ = [ML]/[M][L]
ML + L ⇌ ML₂ K₂ = [ML₂]/[ML][L]
Overall formation constant: β₂ = K₁ × K₂ = [ML₂]/[M][L]²
Factors Affecting Stability:
- Chelate effect: Polydentate ligands form more stable complexes
- Charge density: Higher charge/size ratio increases stability
- Electronic factors: Crystal field effects influence stability
Spectroscopic Properties and Color
The relationship between crystal field splitting and color can be quantified:
Energy-wavelength relationship: E = hc/λ = Δ
Example: [Ti(H₂O)₆]³⁺ absorbs at 500 nm
- E = (6.626 × 10⁻³⁴ J·s)(3 × 10⁸ m/s)/(500 × 10⁻⁹ m)
- E = 3.98 × 10⁻¹⁹ J per photon
- This corresponds to Δ₀ for the d¹ system
Practice Problem Set 1: Oxidation States and Formulas
- Determine the oxidation state of the central metal in:
a) [Pt(NH₃)₂Cl₄]
b) K₂[PdCl₄]
c) [Co(en)₃]Cl₃ - Write the formula for:
a) Hexaamminecobalt(III) sulfate
b) Potassium hexacyanoferrate(II)
c) Dichlorobis(ethylenediamine)platinum(IV) chloride
Solutions:
- a) Pt: +4, b) Pd: +2, c) Co: +3
- a) [Co(NH₃)₆]₂(SO₄)₃, b) K₄[Fe(CN)₆], c) [PtCl₂(en)₂]Cl₂
Practice Problem Set 2: Magnetic Properties
- Predict the magnetic behavior of:
a) [Fe(H₂O)₆]²⁺ (high spin)
b) [Co(NH₃)₆]³⁺ (low spin)
c) [Cu(H₂O)₄]²⁺ - Calculate the magnetic moment for [Cr(H₂O)₆]³⁺.
Solutions:
- a) Paramagnetic (4 unpaired e⁻), b) Diamagnetic (0 unpaired e⁻), c) Paramagnetic (1 unpaired e⁻)
- μ = √[3(3+2)] = √15 = 3.87 BM
Common Error Alert:
When calculating oxidation states, students often forget that the coordination sphere has its own charge separate from counter ions. Always identify what’s inside the brackets first, then consider the counter ions.
Chemistry Check: For the complex [Cr(H₂O)₄Cl₂]Cl, determine: (1) the oxidation state of Cr, (2) the coordination number, (3) possible geometric isomers, and (4) whether it’s likely to be colored.
9: Advanced Topics and Current Research
As we venture into the cutting-edge realm of coordination chemistry, we discover that this field is far from being a completed chapter in chemistry textbooks. Instead, it’s a vibrant, rapidly evolving discipline that’s pushing the boundaries of materials science, medicine, environmental remediation, and even quantum computing. Let’s explore how modern coordination chemistry is shaping the future.
Supramolecular Coordination Chemistry – Beyond Single Molecules
Imagine coordination chemistry as not just individual molecular buildings, but entire cities where coordination compounds interact to create structures and functions that are greater than the sum of their parts. This is the realm of supramolecular coordination chemistry.
Metal-Organic Frameworks (MOFs): These are crystalline materials built from metal ions connected by organic ligands, creating structures with enormous internal surface areas – some have surface areas equivalent to a football field per gram of material!
Applications of MOFs:
- Gas storage: Hydrogen for fuel cells, CO₂ for carbon capture
- Drug delivery: Controlled release of medications
- Catalysis: Single-site catalysts with unprecedented selectivity
- Sensing: Detection of explosives, pollutants, or biomarkers
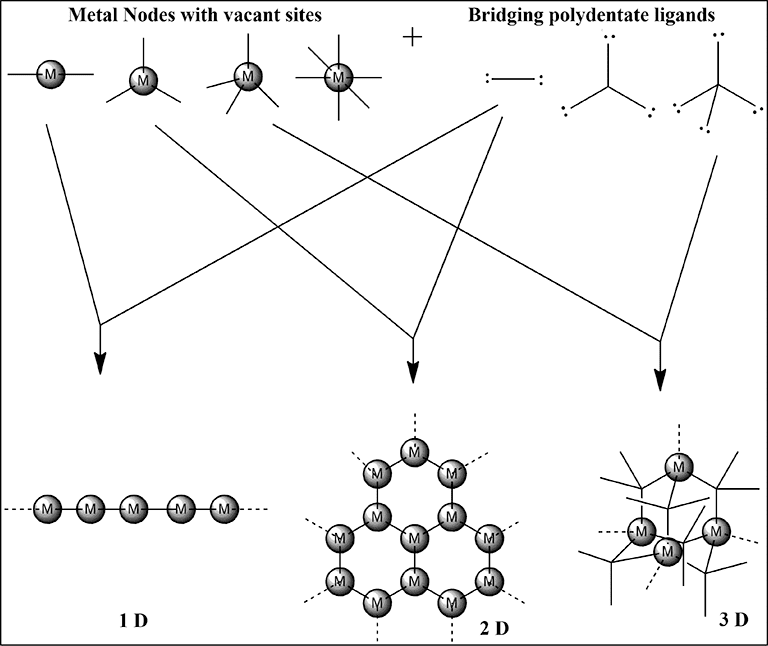
Coordination Cages and Containers: Scientists now design coordination compounds that form hollow structures capable of encapsulating guest molecules. These molecular containers can:
- Selectively bind specific molecules
- Protect reactive species
- Enable reactions in confined spaces with unique selectivity
Single-Molecule Magnets – The Ultimate Miniaturization
Imagine storing digital information on individual molecules – this is becoming reality with single-molecule magnets (SMMs). These coordination compounds exhibit magnetic bistability, meaning they can exist in two different magnetic states, essentially acting as molecular bits.
Key Features of SMMs:
- Magnetic anisotropy: Preferential magnetization direction
- Quantum tunneling: Magnetic relaxation via quantum effects
- Temperature dependence: Performance improves at low temperatures
Potential Applications:
- Ultra-high density data storage: Millions of times denser than current technology
- Quantum computing: Qubits based on molecular spin states
- Molecular spintronics: Electronics based on electron spin
Photocatalysis and Artificial Photosynthesis
One of the most exciting frontiers involves using coordination compounds to mimic and improve upon photosynthesis – nature’s way of converting sunlight into chemical energy.
Ruthenium-Based Photosensitizers: Complexes like [Ru(bpy)₃]²⁺ can absorb visible light and transfer energy to drive chemical reactions. These systems are being developed for:
- Solar fuel production: Converting CO₂ and H₂O to methanol or hydrogen
- Water splitting: Generating hydrogen fuel from water using sunlight
- CO₂ reduction: Converting greenhouse gas to useful chemicals
Mechanism of Photocatalytic Water Splitting:
- Light absorption: Photosensitizer absorbs photon
- Charge separation: Electron promoted to excited state
- Electron transfer: Excited electron reduces water to H₂
- Hole transfer: Oxidizing equivalent splits water to O₂
Current Research Challenges:
- Catalyst stability: Preventing decomposition under harsh conditions
- Efficiency optimization: Maximizing light-to-fuel conversion
- Cost reduction: Developing earth-abundant metal catalysts
Bioinorganic Chemistry – Life’s Coordination Complexes
Modern research is revealing increasingly sophisticated roles for coordination compounds in biological systems.
Metalloenzymes: Over one-third of all enzymes contain metal coordination centers, and we’re discovering new mechanisms constantly:
Nitrogenase: This enzyme converts atmospheric nitrogen to ammonia using a complex iron-molybdenum cluster. Understanding its mechanism could revolutionize fertilizer production.
Photosystem II: The water-oxidizing complex contains a Mn₄CaO₅ cluster that performs one of the most challenging reactions in chemistry – splitting water using light energy.
Artificial Metalloenzymes: Scientists are now creating hybrid catalysts that combine the selectivity of enzymes with the robustness of synthetic coordination compounds.
Coordination Chemistry in Medicine – Precision and Personalization
Targeted Drug Delivery: Modern coordination compounds can be designed to:
- Release drugs only in specific cellular environments
- Target particular types of cancer cells
- Cross the blood-brain barrier for neurological treatments
Theranostics: Combining therapy and diagnostics in single coordination compounds:
- MRI contrast agents that also deliver drugs
- Radiopharmaceuticals that image tumors and provide radiation therapy
- Fluorescent probes that diagnose and treat simultaneously
Photodynamic Therapy: Coordination compounds that generate reactive oxygen species when illuminated, selectively killing cancer cells while sparing healthy tissue.
Green Coordination Chemistry
Environmental consciousness is driving innovation in coordination chemistry:
Sustainable Catalysis:
- Earth-abundant metals: Replacing rare metals with iron, nickel, copper
- Aqueous reactions: Using water instead of organic solvents
- Renewable feedstocks: Converting biomass to chemicals
Environmental Remediation:
- Metal extraction: Removing toxic metals from contaminated water
- Air purification: Catalytic destruction of pollutants
- Plastic degradation: Breaking down polymer waste
Quantum Applications
Coordination compounds are finding applications in the emerging field of quantum technologies:
Quantum Dots: Semiconductor nanocrystals with coordination compound-like properties for:
- Quantum displays: More vivid colors with better efficiency
- Solar cells: Enhanced light absorption and charge separation
- Quantum computing: Information processing using quantum states
Molecular Qubits: Individual coordination compounds serving as quantum bits for quantum computers.
Current Research Frontiers: Scientists at leading universities are developing coordination compounds that can repair themselves when damaged, mimicking biological healing processes. Other researchers are creating compounds that change their properties in response to light, temperature, or pH, enabling “smart” materials that adapt to their environment.
Computational Coordination Chemistry
Modern research heavily relies on computational methods to:
Predict Properties: Before synthesizing new compounds, computers can predict:
- Stability and reactivity
- Electronic and magnetic properties
- Catalytic activity
- Toxicity and environmental impact
Design Principles: Machine learning is helping identify patterns that lead to desired properties, accelerating discovery of new coordination compounds.
High-Throughput Screening: Computers can virtually test thousands of potential coordination compounds, identifying the most promising candidates for experimental work.
Chemistry Check: Consider the future applications we’ve discussed. Which area of coordination chemistry research do you think will have the greatest impact on society in the next 20 years? What challenges need to be overcome to realize these applications?
Future Directions
The future of coordination chemistry lies at the intersection of multiple disciplines:
Coordination Biology: Understanding how evolution has optimized metal-based catalysts
Coordination Materials: Creating materials with unprecedented properties
Coordination Medicine: Developing personalized treatments based on individual genetic profiles
Coordination Sustainability: Solving environmental challenges through better catalysts and materials
Real-World Chemistry Callout:
The coordination compounds being developed today might power the electric vehicles of tomorrow, enable ultra-fast quantum computers, or provide personalized cancer treatments. The fundamental principles you’re learning in CBSE Class 12 are the same ones driving these revolutionary applications.
10: Exam Strategy and Mastery Techniques
Success in CBSE Class 12 Chemistry examinations requires more than just understanding concepts – it demands strategic preparation, efficient problem-solving techniques, and the ability to apply knowledge under exam pressure. Think of this section as your personal coaching session with an experienced chemistry teacher who has guided countless students to excellence.
Understanding the CBSE Examination Pattern
Unit 5 Weightage and Question Distribution:
Coordination Compounds typically carries 3-5 marks in the CBSE Chemistry examination, but its importance extends beyond marks because concepts from this unit often integrate with other topics like chemical bonding and d-block elements.
Expected Question Types:
- 1-mark questions: Definition-based, nomenclature, basic concepts
- 2-mark questions: Oxidation states, coordination numbers, simple comparisons
- 3-mark questions: Structural analysis, isomerism, property predictions
- 5-mark questions: Complete analysis of coordination compounds, including bonding theories
Strategic Study Planning
Phase 1: Foundation Building (Week 1-2)
- Master basic terminology and definitions
- Practice writing formulas and names systematically
- Understand Werner’s theory thoroughly
- Create concept maps linking different topics
Phase 2: Theory Integration (Week 3-4)
- Study VBT and CFT in detail
- Practice predicting magnetic properties
- Understand color and spectral properties
- Work through isomerism systematically
Phase 3: Application and Problem-Solving (Week 5-6)
- Solve numerical problems involving calculations
- Practice case study questions
- Work on previous year questions
- Time yourself while solving problems
Phase 4: Revision and Fine-tuning (Week 7-8)
- Quick revision using summary notes
- Focus on frequently asked topics
- Practice writing complete answers within time limits
- Address any remaining weak areas
Process Analysis: Exam Question Approach
Step 1: Read Carefully: Underline key terms and identify what exactly is being asked
Step 2: Plan Your Answer: Organize your thoughts before writing
Step 3: Use Proper Terminology: Employ correct chemical nomenclature and terminology
Step 4: Show Your Working: For calculations, show all steps clearly
Step 5: Review: Check your answer for completeness and accuracy
High-Yield Topics for CBSE Exams
Based on analysis of previous years’ papers, certain topics appear more frequently:
Must-Know Topics (High Probability):
- Nomenclature: Both naming compounds and writing formulas
- Werner’s theory: Primary and secondary valencies
- Oxidation states: Calculation and determination
- Isomerism: Particularly geometric isomerism in square planar and octahedral complexes
- VBT applications: Predicting magnetic behavior
- Ligand classification: Monodentate, bidentate, chelating agents
Important Topics (Medium Probability):
- Crystal Field Theory: Basic concepts and applications
- Color in coordination compounds: Relationship with d-d transitions
- Stability of complexes: Factors affecting stability
- Applications: Biological and industrial importance
Practice Problem Set 3: Exam-Style Questions
Question 1 (1 mark): Name the following coordination compound: K₄[Fe(CN)₆]
Question 2 (2 marks): What are ambidentate ligands? Give one example.
Question 3 (3 marks): Draw the structures of geometrical isomers of [Co(NH₃)₄Cl₂]⁺. Which of these would show optical isomerism?
Question 4 (5 marks): For the coordination compound [Co(en)₂Cl₂]Cl:
a) Identify the central metal ion and its oxidation state
b) What is the coordination number?
c) Name the compound using IUPAC rules
d) Draw the possible geometric isomers
e) Predict the magnetic behavior using VBT
Model Answers and Marking Schemes
Answer 1: Potassium hexacyanoferrate(II) [1 mark]
Answer 2:
Ambidentate ligands are those which can coordinate to the central metal atom through two different atoms [1 mark].
Example: NO₂⁻ can coordinate through N (nitro) or O (nitrito) [1 mark].
Answer 3:
![Structures of cis and trans isomers of [Co(NH₃)₄Cl₂]⁺] [2 marks for correct structures](https://solvefyai.com/wp-content/uploads/2025/09/image-396.png)
The cis isomer can show optical isomerism as it lacks a plane of symmetry [1 mark].
Answer 4:
a) Central metal: Co, Oxidation state: +3 [1 mark]
b) Coordination number: 6 [1 mark]
c) dichlorobis(ethylenediamine)cobalt(III) chloride [1 mark]
d) [Diagram showing cis and trans isomers] [1 mark]
e) Co³⁺ (d⁶), strong field ligands → low spin → diamagnetic [1 mark]
Common Mistakes and How to Avoid Them
Mistake 1: Incorrect Oxidation State Calculation
- Error: Forgetting to consider the charge on the coordination sphere
- Solution: Always use charge balance method systematically
Mistake 2: Wrong Nomenclature
- Error: Incorrect order of ligands or wrong metal name in anionic complexes
- Solution: Follow IUPAC rules step by step, practice regularly
Mistake 3: Isomerism Confusion
- Error: Missing possible isomers or incorrect structures
- Solution: Draw structures systematically, check for symmetry elements
Mistake 4: Magnetic Property Prediction
- Error: Incorrect application of crystal field theory
- Solution: Identify ligand field strength first, then apply electron pairing rules
Memory Aids and Mnemonics
For Spectrochemical Series: “I Brought Chlorine For Our Water; Nobody Expected Nice Carbonyls”
(I⁻ < Br⁻ < Cl⁻ < F⁻ < OH⁻ < H₂O < NH₃ < en < NO₂⁻ < CN⁻ < CO)
For Common Ligand Names:
- “Aqua-NH₃”: Water and ammonia (neutral ligands keep names)
- “Chloro-Cyano”: Chloride and cyanide (anions end in -o)
For Geometry:
- “2-Linear, 4-Square/Tetra, 6-Octa”: Common coordination numbers and geometries
Chemistry Check: Time yourself solving this: For [Cr(H₂O)₄Cl₂]Cl, determine the oxidation state of Cr, name the compound, and predict whether it will show geometric isomerism. You should complete this in under 3 minutes.
Building Confidence for Success
Visualization Techniques: Before the exam, visualize yourself successfully answering coordination chemistry questions. This mental rehearsal builds confidence and reduces anxiety.
Practice Under Pressure: Regularly solve questions with time limits to simulate exam conditions.
Positive Self-Talk: Replace “I can’t remember” with “I know I’ve studied this thoroughly.”
Strategic Guessing: If unsure, use elimination techniques and chemical logic to make educated guesses.
Final Encouragement: Remember that coordination chemistry is logical and systematic. Every concept builds on previous knowledge, and with proper preparation, you can master this beautiful topic and excel in your CBSE examination.
Conclusion: Mastering Coordination Chemistry for Lifelong Success
As we reach the end of our comprehensive journey through the fascinating world of coordination compounds, it’s time to reflect on what you’ve learned and prepare for both your immediate academic goals and your future scientific endeavors. This conclusion isn’t just a summary – it’s your roadmap to transforming theoretical knowledge into practical mastery and exam success.
Synthesis of Key Concepts
Throughout this guide, we’ve discovered that coordination chemistry is far more than an abstract academic topic. It’s the molecular foundation that makes life possible, from the hemoglobin carrying oxygen in your blood to the chlorophyll enabling photosynthesis in plants. We’ve seen how Alfred Werner’s revolutionary insights in the early 1900s laid the groundwork for understanding these complex molecular architectures, and how modern scientists are pushing the boundaries with applications in medicine, environmental science, and advanced materials.
The beauty of coordination chemistry lies in its logical structure. Every concept builds systematically on previous knowledge:
- Werner’s theory provides the foundational framework
- Nomenclature rules give us a universal language
- Bonding theories (VBT and CFT) explain properties and behavior
- Isomerism reveals the subtle complexities of three-dimensional molecular architecture
- Applications demonstrate the real-world importance of these theoretical concepts
Key Takeaways for CBSE Success
Master the Fundamentals: Your success begins with a solid understanding of basic concepts. Ensure you can quickly identify central metals, determine oxidation states, and classify ligands. These skills form the foundation for solving more complex problems.
Think Systematically: Approach every coordination chemistry problem with a systematic method. Use the MORSE approach (Metal, Oxidation state, Rules, Structure, Electrons) to organize your thinking and avoid careless errors.
Practice Regularly: Coordination chemistry requires active practice, not passive reading. Work through problems daily, focusing on areas where you feel less confident. Remember that understanding comes through doing, not just reading.
Connect Theory to Reality: Whenever you encounter a concept, try to connect it to real-world applications. This not only makes the material more interesting but also helps with retention and deeper understanding.
Visualize in Three Dimensions: Coordination compounds exist in three-dimensional space. Practice drawing structures and visualizing geometric relationships. This skill is crucial for understanding isomerism and predicting properties.
Exam Strategy Recap
Time Management: Coordination compounds questions typically appear in the middle portion of the chemistry paper. Allocate your time based on mark distribution, but don’t spend excessive time on any single question.
Show Your Work: For calculation-based questions, always show your step-by-step approach. Partial credit is often awarded for correct methods, even if the final answer is incorrect.
Use Proper Terminology: Chemistry is a precise science with specific vocabulary. Use correct IUPAC nomenclature and chemical terminology to demonstrate your understanding.
Check for Reasonableness: After solving problems, quickly check if your answers make chemical sense. Are oxidation states reasonable? Do your predicted properties align with known trends?
Building Long-Term Chemical Intuition
Pattern Recognition: As you work with more coordination compounds, you’ll begin to recognize patterns in stability, color, magnetic behavior, and reactivity. Trust these patterns while remaining open to exceptions.
Cross-Unit Connections: Coordination chemistry connects to many other chemistry topics. Strong performance in this unit will enhance your understanding of chemical bonding, periodic trends, and reaction mechanisms.
Scientific Thinking: The logical approach you develop studying coordination chemistry – forming hypotheses, analyzing evidence, drawing conclusions – will serve you well in all scientific endeavors.
Future Pathways and Career Connections
If coordination chemistry has captured your interest, consider these potential career paths:
Research Chemistry: Developing new coordination compounds for applications in catalysis, medicine, or materials science
Biochemistry: Studying metal-containing enzymes and their roles in biological processes
Materials Science: Creating advanced materials with unique properties based on coordination principles
Environmental Science: Using coordination chemistry to address pollution and sustainability challenges
Medicine: Developing new drugs or diagnostic agents based on coordination compounds
Research and Extension Opportunities
For students interested in going beyond the CBSE syllabus:
Extended Reading: Explore advanced topics like organometallic chemistry, bioinorganic chemistry, or supramolecular coordination chemistry
Laboratory Experience: Seek opportunities to work with coordination compounds in school or college laboratories
Science Fairs: Design projects investigating the properties or applications of coordination compounds
Online Resources: Follow current research through scientific journals and educational websites
Final Chemistry Check: As you prepare for your exams, ask yourself: Can I explain to a friend why blood is red, how photosynthesis works, and why certain cancer drugs are effective? If you can connect coordination chemistry to these real-world phenomena, you’ve truly mastered the subject.
Words of Encouragement
Remember that every chemistry student faces challenges when learning coordination chemistry. The key to success is persistent effort, systematic study, and maintaining curiosity about the molecular world around you. The concepts that seem difficult today will become intuitive tools tomorrow.
Your journey through coordination chemistry is part of a larger adventure in understanding the natural world at the molecular level. Every problem you solve, every concept you master, and every connection you make between theory and application builds your scientific literacy and prepares you for future challenges.
Final Thoughts: Beyond the Examination
While your immediate goal may be succeeding in the CBSE Class 12 examination, remember that the knowledge and skills you’re developing have value far beyond any test. You’re learning to think like a scientist, to analyze complex systems, and to understand the molecular basis of life itself.
The coordination compounds you study today are actively working in your body, enabling the photosynthesis that feeds our planet, and serving as the basis for new technologies that will shape our future. By mastering this beautiful topic, you’re connecting yourself to one of the most fundamental and practically important areas of chemistry.
As you move forward, carry with you not just the facts and formulas, but the wonder and curiosity that make science exciting. The molecular world is vast and full of mysteries waiting to be explored. Your solid foundation in coordination chemistry is your passport to participating in that exploration.
Success in coordination chemistry – and in life – comes not from memorizing facts, but from understanding principles, making connections, and applying knowledge creatively to solve new challenges. You have all the tools you need for success. Now go forward with confidence and achieve the excellence you’ve worked so hard to attain.
Recommended –


1 thought on “CBSE Class 12 Chemistry Unit 5: Coordination Compounds Notes, NCERT Solutions & Revision for Board Exams”