CBSE Class 12 Chemistry Unit 4: d and f Block Elements Notes, NCERT Solutions & Revision for Board Exams
Have you ever wondered why iron rusts while gold remains eternally shiny? Or why copper wires conduct electricity so well while aluminum doesn’t tarnish easily? The answers lie in the fascinating world of d and f block elements – the transition metals and inner transition metals that make our modern world possible. From the steel in skyscrapers to the rare earth magnets in your smartphone, these elements are everywhere around you.
Welcome to your comprehensive journey through CBSE Class 12 Chemistry Unit 4, where we’ll explore the captivating chemistry of d and f block elements. Whether you’re preparing for your board exams or simply curious about the elements that power our technology, this guide will transform complex concepts into clear, understandable knowledge.
Learning Objectives
By the end of this study guide, you will be able to:
- Classify and identify d and f block elements based on their electronic configurations and position in the periodic table
- Analyze and predict the physical and chemical properties of transition metals and their compounds
- Explain the formation and properties of coordination compounds using various theories
- Calculate and solve problems related to oxidation states, magnetic properties, and complex formation
- Connect theoretical concepts to real-world applications in industry, medicine, and technology
- Master exam techniques for solving CBSE board questions on d and f block elements
1. Introduction to d and f Block Elements: The Periodic Table’s Powerhouses
Imagine the periodic table as a grand apartment building. While the s and p block elements occupy the corner apartments with simple, predictable layouts, the d and f block elements live in the penthouse suites – complex, fascinating, and full of surprises.
The d block elements, also known as transition metals, occupy the central region of the periodic table from groups 3 to 12. These elements are characterized by the progressive filling of d orbitals in their atoms or ions. The f block elements, consisting of lanthanides and actinides, are even more exclusive – they’re like the VIP floors of our periodic table building.

Electronic Configuration Patterns
The beauty of d block elements lies in their electronic configurations. Unlike s and p block elements that follow straightforward filling patterns, d block elements show interesting exceptions due to the relative energies of s and d orbitals.
For the first transition series (Sc to Zn), the general electronic configuration is [Ar] 3d¹⁻¹⁰ 4s¹⁻². However, nature loves stability, and you’ll encounter fascinating exceptions like:
- Chromium: [Ar] 3d⁵ 4s¹ (instead of 3d⁴ 4s²)
- Copper: [Ar] 3d¹⁰ 4s¹ (instead of 3d⁹ 4s²)
These exceptions occur because half-filled and completely filled d orbitals provide extra stability – think of it as nature’s preference for symmetry and completeness.
Common Error Alert: Students often forget that when writing electronic configurations of transition metal ions, electrons are removed from the 4s orbital first, not the 3d orbital. For example, Fe²⁺ is [Ar] 3d⁶, not [Ar] 3d⁵ 4s¹.
2. General Properties of d Block Elements: The Versatile Performers
Transition metals are like the Swiss Army knives of the periodic table – versatile, reliable, and incredibly useful. Their unique properties stem from their electronic structure and make them indispensable in our daily lives.
Physical Properties That Set Them Apart
Metallic Character and Conductivity
All d block elements are metals with high electrical and thermal conductivity. This property makes copper essential for electrical wiring and silver valuable for high-end electronics. The delocalized electrons in their metallic bonding create an “electron sea” that allows easy movement of charge and heat.
Density and Mechanical Properties
Transition metals are generally dense, hard, and possess high tensile strength. This combination makes them perfect for construction (iron and steel), aerospace applications (titanium), and precision instruments (tungsten). The strong metallic bonding and compact crystal structures contribute to these properties.
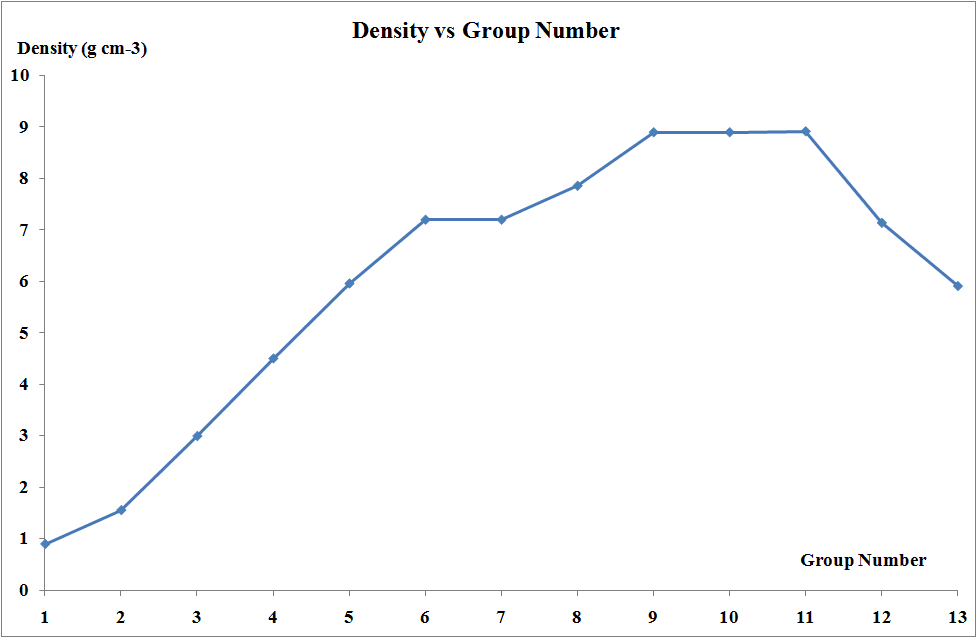
One of the most striking features of transition metals is their ability to form colored compounds. This property arises from d-d electronic transitions when light hits the compound. Different ligands and oxidation states produce different colors, making transition metal chemistry visually spectacular.
Real-World Chemistry: The beautiful blue of copper sulfate crystals, the deep purple of potassium permanganate, and the vibrant green of chromium compounds all demonstrate this property. Gemstones like emeralds (chromium-containing beryl) and rubies (chromium-containing aluminum oxide) owe their colors to transition metal impurities.
Variable Oxidation States: The Shape-Shifters
Unlike main group elements that typically show one or two oxidation states, transition metals are true shape-shifters. Manganese, for instance, can exist in oxidation states from +2 to +7, each with distinct properties and applications.
The variable oxidation states arise because:
- The energy difference between (n-1)d and ns orbitals is relatively small
- Both d and s electrons can participate in bonding
- Different numbers of electrons can be removed without dramatically changing the atom size
Chemistry Check: Can you explain why scandium shows only +3 oxidation state while manganese shows multiple oxidation states from +2 to +7?
3. First Transition Series: From Scandium to Zinc
The first transition series (Sc to Zn) serves as our introduction to transition metal behavior. Each element in this series adds one more electron to the 3d orbital, creating a fascinating progression of properties.
Scandium (Sc): The Lightweight Champion
Scandium, with its [Ar] 3d¹ 4s² configuration, behaves more like a main group element than a typical transition metal. It shows only +3 oxidation state and forms colorless compounds due to the absence of d-d transitions in Sc³⁺ ([Ar] configuration).
Current Research: Scandium is gaining attention in aerospace applications due to its low density and high strength when alloyed with aluminum.
Titanium (Ti): The Corrosion-Resistant Wonder
Titanium’s unique combination of strength, low density, and exceptional corrosion resistance makes it invaluable in aerospace, medical implants, and chemical processing equipment. Its ability to form a protective oxide layer (TiO₂) on its surface prevents further corrosion.
Iron (Fe): The Backbone of Civilization
Iron deserves special attention as the most important transition metal in human civilization. Its multiple oxidation states (+2 and +3 being most common) and ability to form various alloys have shaped our technological development.
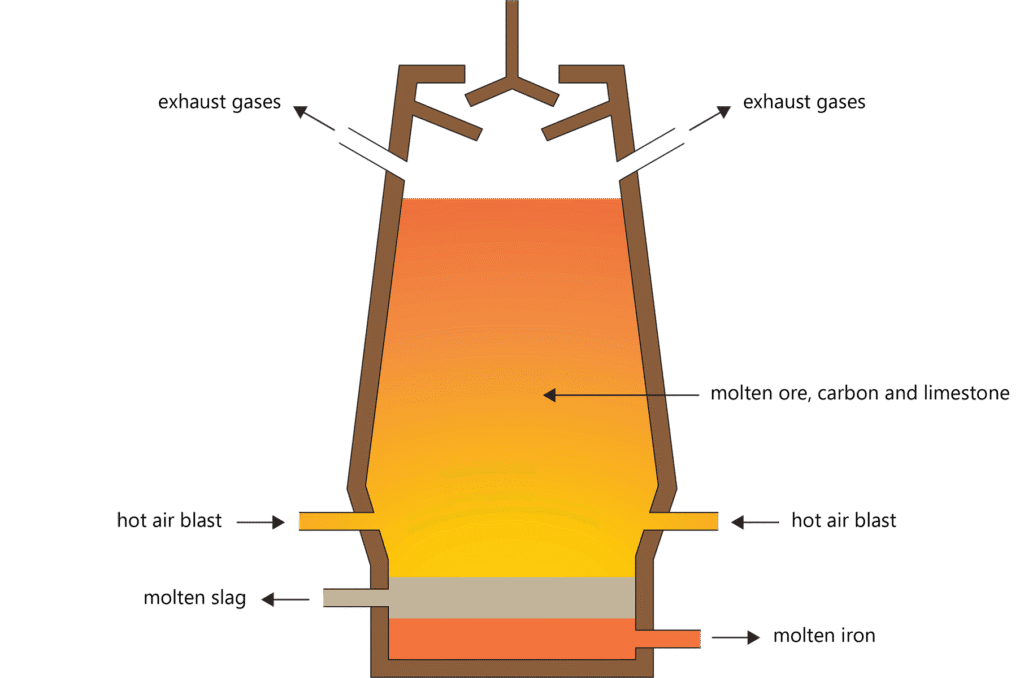
Process Analysis: Iron Extraction from Hematite
- Preparation of raw materials: Iron ore (Fe₂O₃), coke (C), and limestone (CaCO₃)
- Reduction reactions:
- C + O₂ → CO₂
- CO₂ + C → 2CO
- Fe₂O₃ + 3CO → 2Fe + 3CO₂
- Slag formation: CaCO₃ → CaO + CO₂; CaO + SiO₂ → CaSiO₃
- Collection: Molten iron settles at the bottom, slag floats on top
Copper (Cu): The Electrical Conductor
Copper’s exceptional electrical conductivity and malleability make it the preferred choice for electrical applications. Its antimicrobial properties have been recognized for centuries, leading to its use in water pipes and medical applications.
Historical Context: Ancient civilizations recognized copper’s antibacterial properties long before understanding the science behind it. Modern research confirms that copper ions disrupt bacterial cell walls and DNA.
4. Oxidation States and Their Significance
Understanding oxidation states in transition metals is crucial for predicting their chemical behavior and solving numerical problems. Unlike main group elements, transition metals exhibit a wide range of oxidation states due to the availability of both s and d electrons for bonding.
Factors Affecting Oxidation States
Electronic Configuration Influence
The number of unpaired electrons in d orbitals significantly influences the possible oxidation states. Elements with more unpaired d electrons can typically achieve higher oxidation states.
Ionization Energy Trends
The relatively small difference between successive ionization energies in transition metals allows for multiple electron removal. This contrasts with main group elements where ionization energies increase dramatically after removing valence electrons.
[INSERT DIAGRAM: Graph showing variation of oxidation states across the first transition series with maximum oxidation state peaking at Mn]
Stability of Different Oxidation States
Not all oxidation states are equally stable. The stability depends on:
- Ligand field stabilization energy (LFSE)
- Lattice energy in ionic compounds
- Hydration energy in aqueous solutions
- Covalent bonding character
Real-World Chemistry: Permanganate ion (MnO₄⁻) with Mn in +7 oxidation state is a powerful oxidizing agent used in water treatment and analytical chemistry. However, Mn²⁺ is more stable in aqueous solutions, explaining why permanganate readily undergoes reduction.
Calculating Oxidation States: Practice Problems
Problem 1 (MCQ): What is the oxidation state of chromium in K₂Cr₂O₇?
a) +3 b) +6 c) +7 d) +2
Solution:
Let x be the oxidation state of Cr.
2(+1) + 2(x) + 7(-2) = 0
2 + 2x – 14 = 0
2x = 12
x = +6
Answer: b) +6
Common Error Alert: Remember that in compounds like K₂Cr₂O₇, there are two chromium atoms, so the total positive charge from chromium is 2x, not x.
5. Magnetic Properties and Crystal Field Theory
The magnetic behavior of transition metal compounds provides fascinating insights into their electronic structure and bonding. Understanding magnetism also helps explain color, stability, and reactivity patterns.
Types of Magnetic Behavior
Paramagnetic Substances
These substances are attracted to magnetic fields due to the presence of unpaired electrons. Most transition metal compounds are paramagnetic. The magnetic moment can be calculated using the formula:
μ = √[n(n+2)] BM (Bohr Magnetons)
where n is the number of unpaired electrons.
Diamagnetic Substances
Substances with all paired electrons are weakly repelled by magnetic fields. Examples include [Zn(NH₃)₄]²⁺ and [Ni(CN)₄]²⁻.
Ferromagnetic Substances
These show strong attraction to magnetic fields and can be permanently magnetized. Iron, cobalt, and nickel are the classic examples.
Crystal Field Theory: Unlocking the Mystery of Color and Magnetism
Crystal Field Theory (CFT) explains how the presence of ligands affects the energy levels of d orbitals in transition metal complexes. This theory helps us understand:
- Why transition metal compounds are colored
- Why some complexes are paramagnetic while others are diamagnetic
- The stability trends in coordination compounds
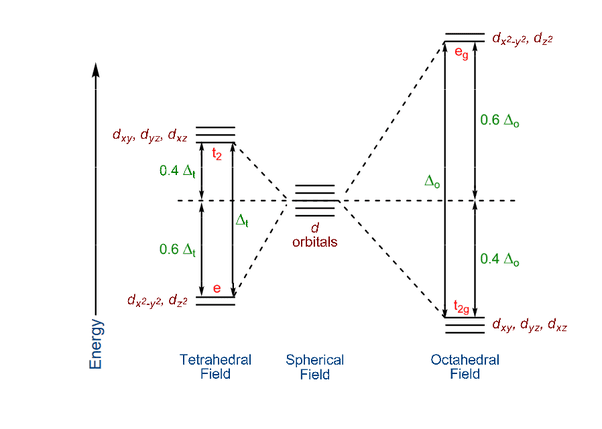
Octahedral Crystal Field Splitting
In an octahedral complex, the five degenerate d orbitals split into two sets:
- t₂g orbitals (dxy, dxz, dyz): lower energy, more stable
- eg orbitals (dx²-y², dz²): higher energy, less stable
The energy difference between these sets is called the crystal field splitting energy (Δₒ).
Process Analysis: Color Formation in Transition Metal Complexes
- Ligand approach: Ligands approach the central metal ion
- Orbital splitting: d orbitals split due to electrostatic interactions
- Electron arrangement: Electrons occupy orbitals according to Hund’s rule and pairing energy
- Light absorption: When light hits the complex, electrons can jump from t₂g to eg orbitals
- Color observation: The complementary color of absorbed light is observed
Chemistry Check: If a complex absorbs light in the blue region (480 nm), what color will you observe? (Hint: Use the color wheel concept)
6. Coordination Compounds: The Architectural Marvels
Coordination compounds represent some of the most beautiful and complex structures in chemistry. These compounds consist of a central metal atom or ion bonded to a number of ligands, creating three-dimensional architectural marvels with precise geometries.
Werner’s Coordination Theory: The Foundation
Alfred Werner’s groundbreaking theory, proposed in 1893, laid the foundation for understanding coordination compounds. His key postulates include:
- Primary valency: The oxidation state of the central metal (satisfied by anions)
- Secondary valency: The coordination number (satisfied by ligands)
- Specific spatial arrangements: Ligands occupy definite positions in space around the central atom
Historical Context: Werner’s theory was revolutionary because it introduced the concept of three-dimensional molecular structures when most chemists thought in terms of simple connectivity. His work earned him the Nobel Prize in Chemistry in 1913.
Classification of Ligands
Monodentate Ligands
These ligands can donate only one pair of electrons to the central metal. Examples include H₂O, NH₃, Cl⁻, and CN⁻.
Bidentate Ligands
These can donate two pairs of electrons from different atoms. Ethylenediamine (en) and oxalate ion (C₂O₄²⁻) are common examples.
Polydentate Ligands
EDTA (ethylenediaminetetraacetic acid) is the most famous hexadentate ligand, capable of donating six pairs of electrons.
Nomenclature Rules for Coordination Compounds
The systematic naming of coordination compounds follows specific IUPAC rules:
- Cation first, anion second
- Within the coordination sphere:
- Ligands before central metal
- Anionic ligands end in ‘-o’
- Neutral ligands use their molecular names (except H₂O = aqua, NH₃ = ammine)
- Prefixes indicate number of ligands
- Central metal naming:
- In cationic/neutral complexes: use element name
- In anionic complexes: use ‘-ate’ ending
Example: [Co(NH₃)₄Cl₂]Cl
Name: Tetraamminedichlorocobalt(III) chloride
Isomerism in Coordination Compounds
Coordination compounds exhibit various types of isomerism, making them structurally diverse and chemically interesting.
Structural Isomerism
- Ionization isomerism: [Co(NH₃)₅Br]SO₄ and [Co(NH₃)₅SO₄]Br
- Hydrate isomerism: [Cr(H₂O)₆]Cl₃ and [Cr(H₂O)₅Cl]Cl₂·H₂O
- Coordination isomerism: [Co(NH₃)₆][Cr(CN)₆] and [Cr(NH₃)₆][Co(CN)₆]
Stereoisomerism
- Geometric isomerism: cis and trans forms in square planar and octahedral complexes
- Optical isomerism: existence of non-superimposable mirror images
![cis and trans isomers of [Pt(NH₃)₂Cl₂] showing different spatial arrangements](https://solvefyai.com/wp-content/uploads/2025/09/image-384.png)
7. Important Compounds and Their Applications
Transition metal compounds find extensive applications in various fields, from industrial catalysis to advanced materials and medical treatments.
Potassium Permanganate (KMnO₄): The Purple Powerhouse
KMnO₄ is one of the most important oxidizing agents in chemistry, known for its distinctive deep purple color and powerful oxidizing properties.
Preparation:
The industrial preparation involves several steps:
- Pyrolusite oxidation: MnO₂ + KOH + O₂ → K₂MnO₄
- Disproportionation: 3K₂MnO₄ + 2H₂O → 2KMnO₄ + MnO₂ + 4KOH
Properties and Reactions:
- In acidic medium: MnO₄⁻ + 8H⁺ + 5e⁻ → Mn²⁺ + 4H₂O
- In neutral/alkaline medium: MnO₄⁻ + 4H⁺ + 3e⁻ → MnO₂ + 2H₂O
Real-World Chemistry: KMnO₄ is used in water treatment to remove organic pollutants, in analytical chemistry for redox titrations, and in medicine as an antiseptic.
Potassium Dichromate (K₂Cr₂O₇): The Orange Oxidizer
This bright orange compound is another powerful oxidizing agent with important laboratory and industrial applications.
Key Reactions:
- Preparation of aldehydes: Primary alcohols are oxidized to aldehydes
- Preparation of ketones: Secondary alcohols are oxidized to ketones
- Chromyl chloride test: Detection of chloride ions
Safety Considerations: Dichromate compounds are toxic and carcinogenic, requiring careful handling and disposal procedures.
Coordination Compounds in Medicine
Cisplatin [PtCl₂(NH₃)₂]
This square planar platinum complex revolutionized cancer treatment. Its mechanism involves:
- Hydrolysis: Replacement of chloride ligands with water in the cell
- DNA binding: Formation of cross-links with DNA bases
- Cell death: Disruption of DNA replication and transcription
EDTA in Medicine
EDTA’s ability to chelate metal ions makes it valuable in treating heavy metal poisoning. It forms stable complexes with toxic metals like lead and mercury, facilitating their removal from the body.
8. Lanthanides: The Rare Earth Elements
The lanthanides (atomic numbers 58-71) are often called rare earth elements, though they’re not particularly rare in Earth’s crust. These f-block elements exhibit unique properties due to the progressive filling of 4f orbitals.
Electronic Configuration and Lanthanide Contraction
All lanthanides have the general electronic configuration [Xe] 4f^n 6s², where n varies from 1 to 14. The key feature of lanthanides is the lanthanide contraction – the steady decrease in atomic and ionic radii across the series.
Cause of Lanthanide Contraction:
As nuclear charge increases across the series, the 4f electrons provide poor shielding for the outer electrons. This results in a stronger effective nuclear charge and smaller atomic radii.
Consequences of Lanthanide Contraction:
- Similar ionic radii: La³⁺ and Lu³⁺ have very similar radii despite the 14-electron difference
- Difficult separation: Lanthanides have very similar chemical properties
- Effect on third transition series: Third transition series elements have smaller radii than expected
Properties of Lanthanides
Physical Properties:
- Silvery-white metals with high luster
- Relatively soft and malleable
- High melting and boiling points
- Good electrical conductivity
Chemical Properties:
- Oxidation state: Predominantly +3, with few exceptions (Ce⁴⁺, Eu²⁺, Yb²⁺)
- Basicity: Lanthanide hydroxides are basic, with basicity decreasing across the series
- Complex formation: Lower tendency compared to transition metals due to larger ionic size
Applications of Lanthanides
Technology Applications:
- Neodymium: Permanent magnets in computer hard drives and wind turbines
- Europium: Red phosphors in TV screens and LED lights
- Yttrium: Superconductors and laser materials
- Cerium: Catalytic converters and glass polishing
Current Research: Lanthanides are crucial for green energy technologies, including wind turbines, electric vehicle motors, and energy-efficient lighting.
9. Actinides: The Radioactive Giants
The actinides (atomic numbers 89-103) are all radioactive elements with complex electronic structures and significant applications in nuclear technology.
Electronic Configuration and Properties
Actinides have the general electronic configuration [Rn] 5f^n 7s², but their electronic configurations are more irregular than lanthanides due to the similar energies of 5f, 6d, and 7s orbitals.
Key Differences from Lanthanides:
- Variable oxidation states: Actinides show a wider range of oxidation states
- Larger ionic radii: 5f orbitals are more diffuse than 4f orbitals
- Greater tendency for complex formation: Due to larger size and variable oxidation states
- Radioactivity: All actinides are radioactive
Important Actinides
Uranium (U):
- Nuclear fuel: ²³⁵U undergoes nuclear fission
- Enrichment: Natural uranium contains only 0.7% ²³⁵U
- Applications: Nuclear power generation and weapons
Plutonium (Pu):
- Artificial element: Produced in nuclear reactors
- Fissile material: ²³⁹Pu is used in nuclear weapons and reactors
- Half-life: ²³⁹Pu has a half-life of 24,100 years
Thorium (Th):
- Alternative nuclear fuel: ²³²Th can be converted to fissile ²³³U
- Abundance: More abundant than uranium in Earth’s crust
- Research focus: Potential for safer nuclear reactor designs
Safety Considerations: All actinides require special handling due to their radioactivity and toxicity. Proper containment and disposal procedures are essential.
10. Industrial Applications and Environmental Impact
The d and f block elements have revolutionized modern industry and technology, but their extraction and use also raise important environmental considerations.
Catalysis: The Art of Acceleration
Transition metals are exceptional catalysts due to their ability to:
- Provide multiple oxidation states for different reaction steps
- Form intermediate compounds that lower activation energy
- Adsorb reactants on their surfaces
Important Industrial Catalysts:
Haber Process (Iron catalyst):
N₂ + 3H₂ ⇌ 2NH₃ (ΔH = -92 kJ/mol)
Iron catalysts with promoters (K₂O, Al₂O₃) enable ammonia synthesis at economically viable rates.
Contact Process (Vanadium pentoxide catalyst):
2SO₂ + O₂ ⇌ 2SO₃
V₂O₅ catalyzes sulfur trioxide formation for sulfuric acid production.
Catalytic Converters (Platinum group metals):
- Oxidation: CO + ½O₂ → CO₂
- Reduction: NO + CO → ½N₂ + CO₂
- Hydrocarbon oxidation: CₓHᵧ + O₂ → CO₂ + H₂O
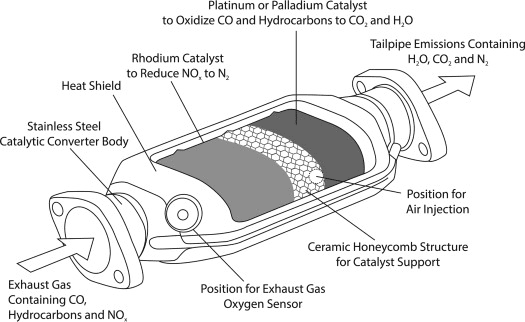
Metallurgy and Alloy Formation
Steel Production:
Iron’s ability to form interstitial compounds with carbon creates various types of steel:
- Mild steel: <0.3% carbon, soft and ductile
- Medium carbon steel: 0.3-0.7% carbon, stronger
- High carbon steel: >0.7% carbon, very hard
Special Alloys:
- Stainless steel: Iron + chromium + nickel (corrosion resistant)
- Brass: Copper + zinc (decorative and musical instruments)
- Bronze: Copper + tin (statues and bearings)
Environmental Considerations
Mining Impact:
- Habitat destruction: Open-pit mining destroys ecosystems
- Water pollution: Acid mine drainage contaminates water sources
- Energy consumption: Metal extraction requires enormous energy input
Recycling Solutions:
- Steel recycling: Reduces energy consumption by 60%
- Copper recycling: Maintains metal quality through multiple cycles
- Rare earth recycling: Critical for sustainable technology development
Green Chemistry Approaches:
- Bioleaching: Using bacteria to extract metals from ores
- Ionic liquids: Environmentally friendly solvents for metal processing
- Urban mining: Recovering metals from electronic waste
11. Exam Preparation Strategies and Problem-Solving Techniques
Success in CBSE Class 12 Chemistry requires strategic preparation and systematic problem-solving approaches. Let’s explore effective techniques for mastering d and f block elements.
High-Yield Topics for Board Exams
Based on previous years’ question patterns, focus your preparation on:
- Electronic configurations of transition metals and their ions (15-20% of questions)
- Oxidation states and their calculations (20-25% of questions)
- Coordination compounds nomenclature and isomerism (25-30% of questions)
- Magnetic properties and crystal field theory (15-20% of questions)
- Industrial applications and important compounds (10-15% of questions)
Question Pattern Analysis
2-Mark Questions:
- Definition-based questions (Werner’s theory, ligands, isomerism)
- Simple calculations (oxidation states, magnetic moments)
- Reason-based questions (Why questions)
3-Mark Questions:
- Explanation of properties and trends
- Nomenclature problems
- Comparison between different series
5-Mark Questions:
- Detailed explanations with examples
- Multiple-step numerical problems
- Process descriptions with chemical equations
Problem-Solving Strategies
Strategy 1: Electronic Configuration Problems
Example Problem: Write the electronic configuration of Mn²⁺ and calculate its magnetic moment.
Step-by-Step Solution:
- Ground state Mn: [Ar] 3d⁵ 4s²
- Remove 2 electrons: Remove from 4s first → Mn²⁺: [Ar] 3d⁵
- Count unpaired electrons: 3d⁵ has 5 unpaired electrons
- Calculate magnetic moment: μ = √[n(n+2)] = √[5(5+2)] = √35 = 5.92 BM
Strategy 2: Coordination Compound Nomenclature
Example Problem: Name the compound [Cr(H₂O)₄Cl₂]Cl·2H₂O
Systematic Approach:
- Identify components: Complex cation + simple anion + water of crystallization
- Name ligands: 4 H₂O = tetraaqua, 2 Cl⁻ = dichloro
- Alphabetical order: dichloro comes before tetraaqua
- Central metal: Chromium with oxidation state calculation: +3
- Complete name: Dichlorotetraaquachromium(III) chloride dihydrate
Strategy 3: Crystal Field Theory Problems
Example Problem: Explain why [Ni(CN)₄]²⁻ is diamagnetic while [NiCl₄]²⁻ is paramagnetic.
Analysis Framework:
- Determine geometry: Both are tetrahedral (d⁸ configuration)
- Identify ligand strength: CN⁻ (strong field) vs Cl⁻ (weak field)
- Apply crystal field splitting:
- [Ni(CN)₄]²⁻: Strong field causes pairing → diamagnetic
- [NiCl₄]²⁻: Weak field allows unpaired electrons → paramagnetic
- Draw electron configurations to support the explanation
Common Mistakes to Avoid
Error 1: Incorrect Electron Removal Order
Wrong: Removing d electrons before s electrons in ions
Correct: Always remove s electrons first when forming cations
Error 2: Forgetting Ligand Alphabetical Order
Wrong: aquadichlorotetraammine…
Correct: dichlorotetraaquaammine…
Error 3: Oxidation State Calculation Errors
Wrong: Not accounting for charge on complex ion
Correct: Always balance total charge including ligands
Error 4: Magnetic Moment Formula Confusion
Wrong: Using μ = √n BM
Correct: Using μ = √[n(n+2)] BM where n = number of unpaired electrons
12. Practice Problems with Detailed Solutions
Let’s test your understanding with a comprehensive set of practice problems covering all major concepts.
Multiple Choice Questions
Question 1: Which of the following shows maximum number of oxidation states?
a) Ti (Z=22) b) V (Z=23) c) Cr (Z=24) d) Mn (Z=25)
Detailed Solution:
Electronic configurations:
- Ti: [Ar] 3d² 4s² → oxidation states: +2, +3, +4
- V: [Ar] 3d³ 4s² → oxidation states: +2, +3, +4, +5
- Cr: [Ar] 3d⁵ 4s¹ → oxidation states: +1, +2, +3, +4, +5, +6
- Mn: [Ar] 3d⁵ 4s² → oxidation states: +2, +3, +4, +5, +6, +7
Manganese can lose all seven electrons (5 from 3d and 2 from 4s), showing the maximum range from +2 to +7.
Answer: d) Mn (Z=25)
Question 2: The magnetic moment of [MnCl₄]²⁻ is:
a) 5.92 BM b) 4.90 BM c) 3.87 BM d) 2.83 BM
Detailed Solution:
- Determine oxidation state of Mn:
Let oxidation state = x
x + 4(-1) = -2
x = +2 - Electronic configuration of Mn²⁺:
Mn: [Ar] 3d⁵ 4s²
Mn²⁺: [Ar] 3d⁵ (5 unpaired electrons) - Calculate magnetic moment:
μ = √[n(n+2)] = √[5(5+2)] = √35 = 5.92 BM
Answer: a) 5.92 BM
Case Study Based Questions
Case Study: Coordination compounds play crucial roles in biological systems. Hemoglobin contains iron in a coordination environment, while chlorophyll contains magnesium. Vitamin B₁₂ contains cobalt as the central metal ion. These compounds demonstrate the importance of coordination chemistry in life processes.
Question 1: What is the oxidation state of iron in hemoglobin where iron is coordinated to four nitrogen atoms of porphyrin ring, one histidine nitrogen, and one oxygen molecule?
Solution:
In oxyhemoglobin:
- Iron is coordinated to 4 N atoms (porphyrin): neutral ligands
- 1 histidine N: neutral ligand
- 1 O₂ molecule: neutral ligand
- Overall complex is neutral
- Therefore, oxidation state of Fe = +2
Question 2: Explain why carbon monoxide is toxic in terms of coordination chemistry.
Solution:
CO toxicity occurs because:
- CO is a stronger ligand than O₂ (stronger σ donor and π acceptor)
- CO binds more readily to Fe²⁺ in hemoglobin than O₂
- CO-hemoglobin complex is more stable than O₂-hemoglobin
- This prevents oxygen transport, leading to asphyxiation
Numerical Problems
Problem 1: Calculate the crystal field stabilization energy (CFSE) for [Ti(H₂O)₆]³⁺ in terms of Δₒ.
Solution:
- Determine electronic configuration: Ti³⁺: [Ar] 3d¹
- Octahedral field splitting: One electron in t₂g orbital
- CFSE calculation:
- t₂g electrons contribute -0.4Δₒ each
- eg electrons contribute +0.6Δₒ each
- CFSE = 1 × (-0.4Δₒ) = -0.4Δₒ
Problem 2: A coordination compound has the molecular formula CrCl₃·6H₂O. When treated with AgNO₃, only two-thirds of the chlorine is precipitated as AgCl. Write the structural formula and IUPAC name.
Solution:
- Analysis: Only 2/3 of Cl⁻ precipitated means only 2 Cl⁻ are ionic
- Structure: [Cr(H₂O)₄Cl₂]Cl·2H₂O
- Verification: 1 Cl⁻ ionic (will precipitate with Ag⁺), but the question states 2/3 are precipitated
- Correct structure: [Cr(H₂O)₄Cl]Cl₂·2H₂O
- IUPAC name: Tetraaquachlorochromium(III) chloride dihydrate
Reasoning-Based Questions
Question: Why do transition metals form colored compounds while main group metals generally form colorless compounds?
Comprehensive Answer:
Transition metals form colored compounds due to d-d electronic transitions:
- Electronic structure: Transition metals have partially filled d orbitals
- Crystal field effect: Ligands cause d orbital splitting into different energy levels
- Light absorption: When light hits the compound, electrons can jump between d orbitals
- Energy matching: The energy gap between split d orbitals often matches visible light energy
- Color observation: We see the complementary color of the absorbed light
Main group metals lack partially filled d orbitals, so they cannot undergo d-d transitions and typically form colorless compounds.
Conclusion: Mastering the Elements of Progress
As we conclude our comprehensive journey through the d and f block elements, reflect on the incredible impact these elements have on our world. From the iron in your blood carrying life-sustaining oxygen to the rare earth elements powering your smartphone, these elements are truly the building blocks of modern civilization.
Key Takeaways for Exam Success
Conceptual Mastery:
- Electronic configurations form the foundation for understanding all other properties
- Crystal field theory explains color, magnetism, and geometry in coordination compounds
- Variable oxidation states arise from the similar energies of s and d orbitals
- Catalytic properties stem from the ability to provide multiple oxidation states and surface adsorption
Problem-Solving Skills:
- Always start with electronic configurations when analyzing properties
- Use systematic approaches for nomenclature and structure determination
- Apply crystal field theory to explain magnetic and color properties
- Connect theoretical concepts to real-world applications
Exam Strategy:
- Time management: Allocate time based on marks (1 mark = 1.5 minutes)
- Answer structure: Start with definitions, provide examples, and conclude with applications
- Diagram importance: Include clear, labeled diagrams for coordination compounds and crystal field splitting
- Units and significant figures: Always include appropriate units in calculations
The Future of d and f Block Elements
As we face global challenges like climate change and sustainable energy, d and f block elements will play crucial roles:
Green Technologies:
- Wind turbines: Neodymium magnets for efficient energy generation
- Electric vehicles: Lithium-ion batteries with transition metal cathodes
- Solar panels: Silver contacts and rare earth elements in photovoltaic cells
- Catalytic converters: Platinum group metals reducing vehicle emissions
Advanced Materials:
- Superconductors: Yttrium-based compounds for efficient power transmission
- Smart alloys: Shape-memory alloys for aerospace and medical applications
- Quantum computers: Rare earth elements in quantum dot technologies
Medical Breakthroughs:
- Cancer treatment: New platinum-based drugs with reduced side effects
- MRI imaging: Gadolinium-based contrast agents for better diagnosis
- Targeted therapy: Coordination compounds for precise drug delivery
Your Path Forward
Whether you’re preparing for board exams, competitive entrance tests, or simply satisfying your scientific curiosity, remember that chemistry is not just about memorizing facts and formulas. It’s about understanding the fundamental principles that govern matter and using that knowledge to improve our world.
Immediate Next Steps:
- Practice regularly: Solve at least 5 problems daily from different topics
- Create connections: Link new concepts to previously learned material
- Teach others: Explaining concepts to classmates reinforces your understanding
- Stay curious: Ask “why” and “how” questions about every phenomenon you encounter
Long-term Learning:
- Read beyond textbooks: Explore recent research in coordination chemistry and materials science
- Laboratory experience: Seek opportunities to work with coordination compounds
- Career exploration: Consider how d and f block elements relate to your future career goals
- Global awareness: Follow developments in sustainable technology and green chemistry
Remember, success in chemistry comes not from cramming facts, but from developing a deep understanding of fundamental principles and their applications. The d and f block elements, with their rich chemistry and diverse applications, offer endless opportunities for exploration and discovery.
As you master these concepts, you’re not just preparing for an exam – you’re building the foundation for understanding the molecular world around you. Whether you become a researcher discovering new materials, an engineer designing sustainable technologies, or simply an informed citizen making better choices about the environment, your understanding of d and f block elements will serve you well.
The elements await your exploration. Let your curiosity guide you, let your understanding deepen with each problem you solve, and remember that every great chemist started exactly where you are now – with questions, determination, and the desire to understand the beautiful complexity of matter.
Your journey with d and f block elements has just begun. Make it count.
This comprehensive study guide provides everything you need to excel in CBSE Class 12 Chemistry Unit 4. Use it as your primary resource, supplement it with NCERT textbook reading, and practice regularly with the included problems. Success in chemistry requires consistent effort, conceptual understanding, and practical application – all of which this guide helps you develop.
Good luck with your preparations, and remember: chemistry is not just a subject to study, but a lens through which to understand and improve our world!
Recommended –