The Dynamic Dance of Earth’s Systems
Picture this: you’re standing on a beach at sunset, watching waves crash against the shore while seagulls soar overhead. What you’re witnessing isn’t just a beautiful scene-it’s the intricate interaction of Earth’s four major systems working together in perfect harmony. The atmosphere provides the wind that creates those waves, the hydrosphere supplies the water, the geosphere forms the rocky coastline, and the biosphere includes everything alive, from the seagulls to the microscopic plankton in the water.
Welcome to AP Environmental Science Unit 4: Earth Systems and Resources, where we’ll explore how our planet operates as an interconnected system of systems. This unit forms the foundation for understanding virtually every environmental issue you’ll encounter, from climate change to resource depletion, making it absolutely crucial for your APES exam preparation.
Understanding Earth’s systems isn’t just about memorizing facts-it’s about recognizing the delicate balance that sustains all life on our planet. When you grasp how the atmosphere, hydrosphere, geosphere, and biosphere interact, you’ll begin to see environmental problems not as isolated issues, but as symptoms of disrupted system interactions. This holistic understanding is exactly what the AP Environmental Science curriculum aims to develop in students like you.
In this comprehensive guide, we’ll journey through each of Earth’s major systems, explore their intricate relationships, and discover how human activities are reshaping these ancient cycles. By the end, you’ll not only be prepared for the AP exam but also equipped with the knowledge to understand and address the environmental challenges facing our world today.
The Four Spheres: Earth’s Interconnected Systems
The Atmosphere: Our Protective Blanket
The atmosphere is far more than just the air we breathe-it’s a complex, layered system that regulates Earth’s temperature, protects us from harmful radiation, and drives weather patterns across the globe. Understanding atmospheric structure and composition is fundamental to grasping climate science and air pollution issues.

Atmospheric Layers and Their Functions
Starting from Earth’s surface, the troposphere extends about 10-15 kilometers upward and contains roughly 75% of the atmosphere’s mass. This is where all weather occurs, where we live and breathe, and where most air pollution accumulates. The temperature decreases with altitude in this layer, creating the convection currents that drive weather systems.
Above the troposphere lies the stratosphere, home to the crucial ozone layer. Here, ozone (O₃) molecules absorb harmful ultraviolet radiation, protecting life on Earth’s surface. The temperature actually increases with altitude in this layer due to ozone’s heat-absorbing properties. This temperature inversion prevents vertical mixing, which is why pollutants released into the stratosphere can persist for years.
The mesosphere and thermosphere complete the atmospheric structure, with temperatures decreasing then dramatically increasing with altitude. While these upper layers might seem irrelevant to environmental science, they’re crucial for satellite operations and protection from space debris.
Did You Know? The total mass of Earth’s atmosphere is approximately 5.15 × 10¹⁸ kilograms, but 99% of this mass is contained within just 32 kilometers of the surface!
Atmospheric Composition and Its Environmental Significance
Dry air consists of roughly 78% nitrogen, 21% oxygen, and 1% other gases including argon, carbon dioxide, and trace amounts of other compounds. However, these seemingly small percentages of trace gases have enormous environmental impacts. Carbon dioxide, despite comprising only about 0.04% of the atmosphere, is the primary driver of anthropogenic climate change.

Water vapor, though highly variable in concentration, is actually the most abundant greenhouse gas. Its concentration ranges from nearly 0% in polar regions to up to 4% in tropical areas. This variability makes water vapor both a feedback mechanism and a primary driver of weather patterns.
The Hydrosphere: Water in All Its Forms
Water covers approximately 71% of Earth’s surface and exists in solid, liquid, and gaseous forms throughout the planet. The hydrosphere encompasses all water on Earth, from vast oceans to tiny water droplets in clouds, and its constant movement through the water cycle connects all other Earth systems.
The Global Water Cycle: Nature’s Recycling System
The water cycle represents one of Earth’s most important biogeochemical cycles, constantly moving water between the atmosphere, land surface, and underground reservoirs. Evaporation from oceans, lakes, and rivers transforms liquid water into water vapor, while transpiration from plants adds moisture to the atmosphere through their leaves. Together, these processes are called evapotranspiration.

Condensation occurs when water vapor cools and forms droplets around microscopic particles called condensation nuclei. These droplets grow into clouds and eventually fall as precipitation-rain, snow, sleet, or hail. Once water reaches the ground, it follows several pathways: some immediately evaporates back to the atmosphere, some infiltrates into soil to become groundwater, and some flows over the surface as runoff toward streams, rivers, and eventually oceans.
Groundwater and Surface Water Interactions
Understanding the relationship between groundwater and surface water is crucial for managing water resources sustainably. Groundwater exists in underground formations called aquifers, which are layers of permeable rock, sediment, or soil that can store and transmit water. The water table represents the upper boundary of the saturated zone, where all available spaces in soil and rock are filled with water.
Confined aquifers occur when water-bearing rock layers are sandwiched between impermeable layers, creating pressure that can cause water to rise in wells without pumping. Unconfined aquifers have direct contact with the atmosphere through soil and surface water, making them more susceptible to contamination but also more easily recharged.
The Geosphere: Earth’s Solid Foundation
The geosphere includes all of Earth’s solid materials, from surface soils to the planet’s core. Understanding geological processes is essential for comprehending natural hazards, mineral resources, soil formation, and how human activities interact with Earth’s physical structure.
Plate Tectonics: The Dynamic Earth
Plate tectonics theory explains how Earth’s lithosphere-the rigid outer shell including the crust and upper mantle-is divided into several large and small plates that slowly move over the more fluid asthenosphere below. This movement, driven by heat from Earth’s interior, creates most of the planet’s major geological features and processes.
Divergent boundaries occur where plates move apart, creating new oceanic crust at mid-ocean ridges and rift valleys on continents. The Mid-Atlantic Ridge, for example, spreads about 2-3 centimeters per year, gradually widening the Atlantic Ocean.
Convergent boundaries form where plates collide, creating mountain ranges, deep ocean trenches, and volcanic activity. When oceanic plates collide with continental plates, the denser oceanic plate subducts beneath the continental plate, forming volcanic mountain ranges like the Andes.
Transform boundaries occur where plates slide past each other horizontally, creating earthquake-prone zones like California’s San Andreas Fault. These boundaries neither create nor destroy crust but can generate devastating seismic activity.
Rock Cycle and Mineral Resources
The rock cycle describes the continuous transformation of rocks through geological processes. Igneous rocks form from cooling magma or lava, sedimentary rocks develop from compressed and cemented sediments, and metamorphic rocks result from existing rocks being altered by heat and pressure.
This cycle is crucial for understanding mineral resource formation. Many economically important minerals concentrate during specific stages of the rock cycle. For example, hydrothermal processes associated with igneous activity can create deposits of metals like copper, gold, and silver.
The Biosphere: Life’s Complex Web
The biosphere encompasses all living organisms on Earth and their interactions with the atmosphere, hydrosphere, and geosphere. This sphere demonstrates the incredible adaptability of life and its ability to modify Earth’s other systems.
Ecosystem Structure and Function
Ecosystems represent functional units where organisms interact with each other and their physical environment. Primary producers (autotrophs) form the foundation of all ecosystems by converting inorganic compounds into organic matter through photosynthesis or chemosynthesis. Plants, algae, and certain bacteria serve this crucial role.
Primary consumers (herbivores) feed directly on producers, while secondary consumers (carnivores) eat herbivores. Tertiary consumers occupy the top of food chains, and decomposers break down dead organic matter, returning nutrients to the ecosystem.
Energy flows through ecosystems in one direction, from producers to various consumer levels, with each transfer losing approximately 90% of available energy as heat. This 10% rule explains why food chains rarely exceed four or five levels and why there are typically fewer predators than prey organisms.
Biogeochemical Cycles: Nature’s Recycling Systems
The Carbon Cycle: Climate’s Master Controller
The carbon cycle represents one of Earth’s most important biogeochemical processes, moving carbon through the atmosphere, hydrosphere, geosphere, and biosphere. Understanding this cycle is crucial for comprehending climate change and its impacts.
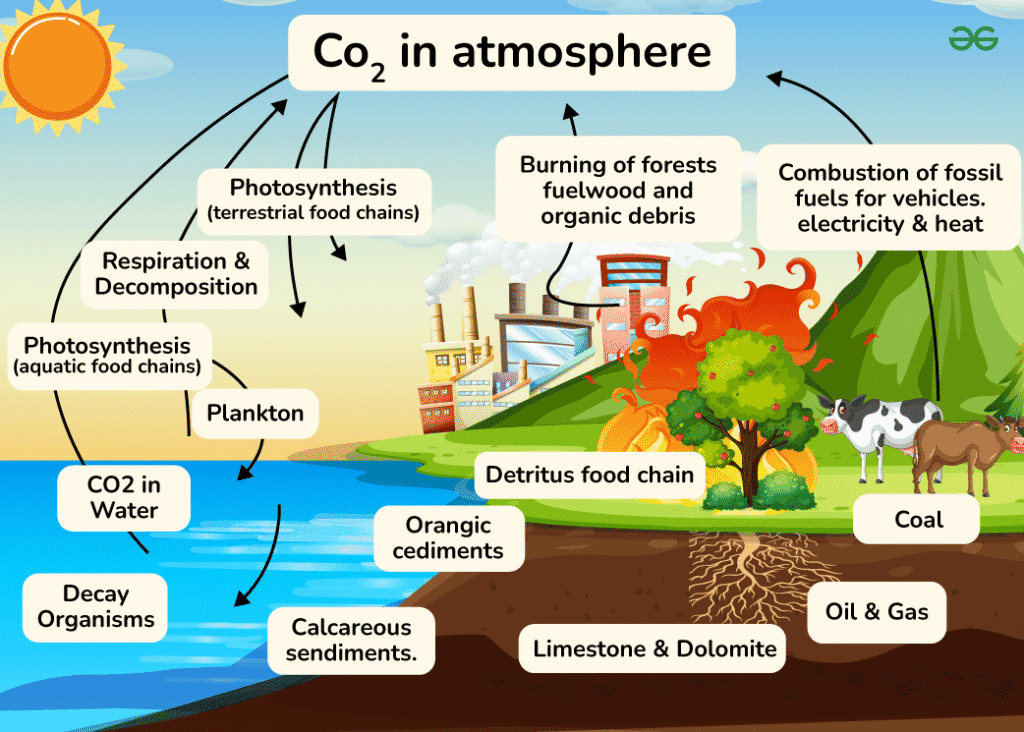
Carbon Reservoirs and Fluxes
Carbon exists in several major reservoirs on Earth. The atmosphere contains carbon primarily as carbon dioxide (CO₂) and methane (CH₄). The hydrosphere stores carbon as dissolved CO₂, carbonic acid, and bicarbonate ions. Living biomass in the biosphere contains carbon in organic compounds, while fossil fuels and sedimentary rocks in the geosphere represent long-term carbon storage.
Photosynthesis removes CO₂ from the atmosphere as plants convert it into glucose and other organic compounds. This process, represented by the equation 6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂, temporarily stores atmospheric carbon in living tissue.
Cellular respiration reverses this process, breaking down organic compounds to release energy and returning CO₂ to the atmosphere. The equation C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + ATP demonstrates how organisms release stored carbon.
Human Impacts on the Carbon Cycle
Human activities have significantly altered the natural carbon cycle, primarily through fossil fuel combustion, deforestation, and land use changes. Burning coal, oil, and natural gas releases carbon that was sequestered millions of years ago, adding approximately 9-10 billion tons of carbon to the atmosphere annually.
Deforestation not only eliminates carbon-absorbing trees but also releases stored carbon when forests are burned or decay. This double impact makes deforestation responsible for roughly 15-20% of annual CO₂ emissions.
The Nitrogen Cycle: Life’s Essential Element
Nitrogen is essential for all living organisms as a component of amino acids, proteins, and nucleic acids. However, most organisms cannot use atmospheric nitrogen (N₂) directly, making the nitrogen cycle crucial for converting nitrogen into biologically available forms.

Nitrogen Fixation and Biological Availability
Nitrogen fixation converts atmospheric N₂ into ammonia (NH₃) or ammonium (NH₄⁺), forms that organisms can use. Certain bacteria, including those living in symbiotic relationships with legume plants, possess the enzyme nitrogenase that catalyzes this energy-intensive process.
Nitrification is a two-step process where soil bacteria first oxidize ammonia to nitrite (NO₂⁻), then other bacteria convert nitrite to nitrate (NO₃⁻). Plants readily absorb both ammonium and nitrate, incorporating nitrogen into organic compounds.
Denitrification completes the cycle by converting nitrates back to nitrogen gas under anaerobic conditions, returning nitrogen to the atmosphere. This process occurs in waterlogged soils, sediments, and groundwater where oxygen is limited.
Human Alterations and Environmental Consequences
Human activities have dramatically increased the amount of biologically available nitrogen through fertilizer production and fossil fuel combustion. The Haber-Bosch process, which produces ammonia for fertilizers, has roughly doubled the amount of fixed nitrogen on Earth.
Excess nitrogen in ecosystems can cause eutrophication in aquatic systems, leading to algal blooms, oxygen depletion, and fish kills. Nitrogen oxides from combustion contribute to acid rain and smog formation, while nitrous oxide (N₂O) acts as a potent greenhouse gas.
The Phosphorus Cycle: The Limiting Factor
Unlike carbon and nitrogen, phosphorus has no significant atmospheric component, making the phosphorus cycle entirely sedimentary. This characteristic often makes phosphorus the limiting nutrient in many ecosystems.
Phosphorus Movement and Availability
Phosphorus primarily exists in rocks as phosphate minerals. Weathering slowly releases phosphates into soil and water, where plants absorb them for growth. Animals obtain phosphorus by eating plants or other animals, and decomposition returns phosphorus to soil and water.
Rivers transport dissolved and particulate phosphorus to oceans, where much of it eventually settles into marine sediments. Over geological time, these sediments may be uplifted to form new rock, completing the cycle.
Human Impact and Resource Depletion
Humans have accelerated phosphorus movement through mining and fertilizer application. Phosphate rock mining provides phosphorus for fertilizers, but these deposits are finite and geographically concentrated, raising concerns about future phosphorus security.
Study Tip: Remember that phosphorus has no gaseous phase under normal Earth conditions, unlike carbon and nitrogen. This makes phosphorus pollution particularly persistent in aquatic systems.
Soil Systems: The Foundation of Terrestrial Life
Soil Formation and Composition
Soil represents one of Earth’s most complex and vital resources, forming at the interface between the geosphere, atmosphere, hydrosphere, and biosphere. Understanding soil systems is crucial for agriculture, ecosystem management, and environmental conservation.
Factors Affecting Soil Formation
The five factors of soil formation-parent material, climate, topography, organisms, and time-interact to create the diverse soil types found across Earth’s surface.
Parent material provides the initial mineral components through rock weathering. Different rocks produce soils with varying chemical and physical properties. For example, soils formed from granite tend to be acidic and well-drained, while those from limestone are typically alkaline and may have drainage issues.
Climate affects both chemical weathering rates and biological activity. Higher temperatures and precipitation generally accelerate soil formation, while extreme conditions like desert heat or arctic cold slow the process significantly.
Topography influences water movement, erosion, and deposition. Steep slopes typically have thin soils due to erosion, while valleys and flat areas often accumulate deeper, more fertile soils.
Organisms, including plants, animals, bacteria, and fungi, contribute organic matter, create soil structure, and drive chemical transformations. Earthworms, for instance, can process tons of soil per acre annually, mixing organic and mineral components.
Time allows all other factors to work together. Soil formation is generally slow, with mature soils requiring hundreds to thousands of years to develop.
Soil Horizons and Profile Development
A mature soil develops distinct horizons-layers with different physical and chemical properties. The O horizon consists of organic matter in various stages of decomposition. The A horizon contains a mixture of organic matter and minerals, giving it a dark color and making it crucial for plant growth.
The B horizon represents the zone of accumulation, where materials leached from upper layers collect. This horizon often has distinct colors due to iron oxides and accumulated clay particles. The C horizon consists of partially weathered parent material, while bedrock forms the foundation beneath all soil layers.
Soil Chemistry and Nutrient Cycling
Soil pH and Nutrient Availability
Soil pH dramatically affects nutrient availability and plant growth. Most nutrients are optimally available in slightly acidic to neutral soils (pH 6.0-7.0), though different plants have varying pH preferences.
Acidic soils (pH < 7) can make aluminum toxic to plants while reducing the availability of essential nutrients like phosphorus, calcium, and magnesium. Alkaline soils (pH > 7) can cause iron, manganese, and zinc deficiencies.
Cation exchange capacity (CEC) measures soil’s ability to hold and exchange positively charged nutrients like calcium, magnesium, and potassium. Clay particles and organic matter provide most of a soil’s CEC, making these components crucial for soil fertility.
Soil Organic Matter and Decomposition
Soil organic matter consists of living organisms, fresh organic debris, and humus-the stable, decomposed organic material that gives soil its dark color and many beneficial properties. Humus improves soil structure, water retention, and nutrient availability while providing slow-release nutrients for plants.
Decomposer organisms, including bacteria, fungi, and various invertebrates, break down organic matter and recycle nutrients. This decomposition process is temperature and moisture dependent, occurring faster in warm, moist conditions and slower in cold or dry environments.
Earth’s Energy Budget and Climate Systems

Solar Radiation and Earth’s Energy Balance
Understanding how Earth receives, absorbs, and redistributes solar energy is fundamental to comprehending climate systems and environmental change patterns.
Solar Input and Atmospheric Interactions
Earth receives approximately 1,360 watts per square meter of solar energy at the top of the atmosphere-a value called the solar constant. However, this energy isn’t distributed evenly across the planet due to Earth’s spherical shape and axial tilt.
About 30% of incoming solar radiation is immediately reflected back to space by clouds, atmospheric particles, and Earth’s surface. This planetary albedo varies significantly among different surfaces: fresh snow reflects up to 90% of incident radiation, while dark forests may reflect only 10%.
The remaining 70% of solar energy is absorbed by the atmosphere and Earth’s surface, warming the planet. This absorbed energy drives weather systems, ocean currents, and the water cycle while maintaining temperatures suitable for life.
The Greenhouse Effect: Natural and Enhanced
The natural greenhouse effect occurs when certain atmospheric gases absorb and re-emit infrared radiation, warming Earth’s surface by approximately 33°C above what it would be without an atmosphere. Water vapor, carbon dioxide, methane, and other greenhouse gases trap outgoing longwave radiation while allowing most incoming shortwave solar radiation to pass through.
The enhanced greenhouse effect results from human activities increasing atmospheric concentrations of greenhouse gases. Carbon dioxide concentrations have increased from approximately 280 ppm in pre-industrial times to over 410 ppm today, primarily due to fossil fuel combustion and deforestation.
Did You Know? Without the natural greenhouse effect, Earth’s average temperature would be about -18°C (0°F), making the planet largely uninhabitable for current life forms.
Climate Patterns and Global Circulation

Atmospheric Circulation Cells
Earth’s climate system is driven by the unequal heating of different latitudes, creating large-scale atmospheric circulation patterns. Near the equator, intense solar heating causes air to rise, cool, and lose moisture as precipitation. This creates the Intertropical Convergence Zone (ITCZ), characterized by heavy rainfall and thunderstorms.
The rising air at the equator eventually descends around 30° north and south latitude, creating subtropical high-pressure zones associated with many of the world’s deserts. Some of this descending air flows back toward the equator as trade winds, completing the Hadley circulation cell.
At higher latitudes, the Ferrel cells and polar cells create additional circulation patterns that help redistribute heat from equatorial regions toward the poles. These circulation patterns strongly influence regional climate conditions and seasonal weather patterns.
Ocean Circulation and Heat Transport
Ocean currents play a crucial role in global heat distribution, transporting warm water from tropical regions toward the poles and returning cold water toward the equator. Surface currents are primarily driven by wind patterns, while deep water currents are driven by density differences caused by temperature and salinity variations.
The thermohaline circulation, often called the “global conveyor belt,” represents the interconnected system of deep and surface currents that circulate throughout the world’s oceans. This system transports enormous amounts of heat and helps moderate global climate patterns.
Changes in ocean circulation can have dramatic climate effects. For example, the El Niño Southern Oscillation (ENSO) periodically alters Pacific Ocean currents, causing significant weather changes across much of the globe.
Real-World Applications and Case Studies
Case Study 1: The Dust Bowl – When Earth Systems Fail
The Dust Bowl of the 1930s provides a powerful example of how human activities can disrupt Earth system interactions with devastating consequences. This environmental disaster resulted from the complex interaction of agricultural practices, climate patterns, and soil systems.
During the 1920s, farmers across the Great Plains plowed millions of acres of native grassland to plant wheat, driven by high grain prices and government incentives. The native grasses had evolved deep root systems that held soil in place and helped it retain moisture during periodic droughts.
When severe drought struck in the early 1930s, the exposed topsoil became vulnerable to wind erosion. Massive dust storms, some carrying soil particles as far as Washington D.C. and New York City, stripped away billions of tons of fertile topsoil. The Black Blizzards of 1934 and 1935 darkened skies across the continent and created some of the most iconic images of environmental destruction in American history.
This disaster demonstrated how the geosphere (soil), atmosphere (wind patterns), hydrosphere (drought conditions), and biosphere (vegetation removal) are interconnected. The lesson learned led to improved agricultural practices, including contour plowing, crop rotation, and shelter belt planting that work with natural systems rather than against them.
Case Study 2: The Aral Sea Disappearance
The Aral Sea crisis represents one of the most dramatic examples of human-induced environmental change, showing how water diversions can collapse entire ecosystem services and affect regional climate systems.
Once the world’s fourth-largest lake, the Aral Sea began shrinking in the 1960s when Soviet irrigation projects diverted its tributary rivers-the Amu Darya and Syr Darya-to grow cotton in the desert. By 2014, the eastern basin had completely dried up, and the entire sea had lost more than 90% of its original area.
This dramatic change created a cascade of environmental problems. The exposed lake bed, contaminated with salt and agricultural chemicals, became a source of dust storms affecting regional air quality and human health. The loss of the large water body altered local climate patterns, making winters colder and summers hotter while reducing precipitation.
The fishing industry collapsed, eliminating jobs for thousands of people, while the increased salinity killed most remaining fish species. Ships that once sailed the Aral Sea now sit stranded in what locals call the “ship graveyard.”
This case study illustrates how human modifications to the hydrosphere can trigger changes throughout all Earth systems, affecting atmosphere (dust storms), geosphere (salt deposits), and biosphere (ecosystem collapse) with lasting socioeconomic consequences.
Case Study 3: Iceland’s Geothermal Success Story
Iceland provides an excellent example of how understanding Earth systems can lead to sustainable resource management. The island nation sits on the Mid-Atlantic Ridge, where tectonic activity creates abundant geothermal energy resources.
Icelanders have learned to harness their position at the intersection of the North American and Eurasian plates, where magma near the surface heats groundwater to create hot springs and geysers. Today, geothermal energy provides about 25% of Iceland’s electricity and heats approximately 90% of homes in the capital city of Reykjavik.
The Hellisheiði Geothermal Power Plant, one of the world’s largest, demonstrates how geosphere processes can be converted into clean energy. The plant not only generates electricity but also captures CO₂ from the geothermal fluids and injects it into basaltic rock formations, where it permanently mineralizes-effectively turning a greenhouse gas into stone.
This approach shows how understanding interactions between Earth systems can create solutions that work with natural processes rather than against them. Iceland’s success has inspired similar projects worldwide, from Kenya’s Rift Valley to the western United States.
Environmental Connections and Current Issues
Climate Change and Earth System Feedbacks
Climate change represents the ultimate example of Earth system interactions, involving complex feedbacks between the atmosphere, hydrosphere, geosphere, and biosphere. Understanding these feedbacks is crucial for predicting future climate conditions and developing effective mitigation strategies.
Positive feedback loops amplify climate changes. As global temperatures rise, Arctic ice melts, reducing the planet’s albedo and causing more solar energy absorption. Similarly, thawing permafrost releases stored carbon dioxide and methane, further enhancing the greenhouse effect.
Negative feedback loops can help stabilize climate systems. Increased atmospheric CO₂ can enhance plant growth in some regions, potentially increasing carbon sequestration. Higher temperatures also increase evaporation, potentially creating more clouds that reflect incoming solar radiation.
However, current research suggests that positive feedbacks are overwhelming negative ones, accelerating climate change beyond what simple greenhouse gas increases would predict.
Resource Depletion and Sustainability
Mineral resource extraction demonstrates how human activities interact with geosphere processes. Many economically important minerals form through geological processes operating over millions of years, making them essentially non-renewable on human timescales.
Peak phosphorus concerns highlight how critical resources can become limiting factors. Unlike fossil fuels, phosphorus has no substitutes for biological functions, and high-grade phosphate rock deposits are geographically concentrated and finite.
Water resource depletion shows how human demands can exceed natural replenishment rates. Many important aquifers, including the Ogallala Aquifer underlying much of the American Great Plains, are being depleted faster than they can recharge, threatening long-term agricultural sustainability.
Pollution and Earth System Disruption
Air pollution demonstrates how local emissions can have global consequences. Persistent organic pollutants (POPs) and mercury can travel through atmospheric circulation to contaminate remote regions, including the Arctic, through a process called global distillation.
Plastic pollution shows how human-made materials can enter and persist in natural cycles. Microplastics have been found in atmospheric precipitation, deep ocean sediments, and even human tissues, demonstrating how synthetic materials can become integrated into Earth system processes.
Acid rain provides an example of how atmospheric chemistry changes can affect terrestrial and aquatic ecosystems. Sulfur dioxide and nitrogen oxides from fossil fuel combustion react with water vapor to form sulfuric and nitric acids, which fall as precipitation with pH values as low as 4.0.
Current Research and Trends
Earth System Science and Global Monitoring
Modern Earth system science takes an integrated approach to studying our planet, recognizing that understanding environmental changes requires examining interactions between all four spheres. This holistic perspective has led to significant advances in environmental monitoring and prediction.
Satellite remote sensing has revolutionized our ability to monitor Earth systems from space. Instruments aboard satellites can measure atmospheric greenhouse gas concentrations, track deforestation, monitor ocean temperatures, and assess soil moisture across the globe. The Landsat program, now in its fifth decade, provides an invaluable long-term record of Earth surface changes.
Global climate models integrate data from all Earth systems to predict future climate conditions. These models have become increasingly sophisticated, incorporating detailed representations of atmospheric chemistry, ocean circulation, vegetation dynamics, and human activities. The latest generation of models can simulate climate processes at spatial resolutions of less than 100 kilometers.
Emerging Technologies and Solutions
Carbon capture and storage (CCS) technologies aim to remove CO₂ from the atmosphere and store it in geological formations. Direct air capture systems use chemical processes to extract CO₂ directly from ambient air, while enhanced weathering accelerates natural rock weathering processes to sequester carbon.
Precision agriculture uses GPS technology, sensors, and data analytics to optimize fertilizer and water application, reducing environmental impacts while maintaining crop yields. These technologies can reduce nitrogen and phosphorus runoff by applying nutrients only where and when they’re needed.
Ecosystem restoration projects are increasingly using scientific understanding of Earth system interactions to restore degraded environments. The Loess Plateau restoration in China demonstrates how reforestation and soil conservation can restore productivity to severely eroded landscapes.
Geoengineering: Technological Interventions in Earth Systems
Solar radiation management proposals aim to reduce incoming solar radiation by injecting reflective particles into the stratosphere or brightening marine clouds. While these approaches might reduce global temperatures, they could have unintended consequences for precipitation patterns and regional climates.
Ocean fertilization experiments investigate whether adding nutrients to ocean regions can enhance phytoplankton growth and increase carbon sequestration. However, results suggest these interventions could disrupt marine food webs and create oxygen-depleted zones.
These geoengineering approaches highlight the complexity of Earth system interactions and the challenges of predicting consequences when intervening in natural processes.
Study Guide Section
Key Concepts and Definitions
Earth Systems Integration
- Atmosphere: The gaseous envelope surrounding Earth, consisting of distinct layers with different temperature and composition profiles
- Hydrosphere: All water on Earth in solid, liquid, and gaseous phases, including oceans, rivers, lakes, groundwater, and atmospheric moisture
- Geosphere: Earth’s solid components, including rocks, minerals, and soils, extending from the surface to the planet’s core
- Biosphere: All living organisms and their interactions with the physical environment
Biogeochemical Cycles
- Carbon Cycle: The movement of carbon through Earth’s systems, including photosynthesis, respiration, decomposition, and fossil fuel combustion
- Nitrogen Cycle: The transformation of nitrogen between atmospheric N₂ and biologically available forms through fixation, nitrification, and denitrification
- Phosphorus Cycle: The movement of phosphorus through rocks, soils, organisms, and water bodies without a significant atmospheric component
- Water Cycle: The continuous movement of water through evaporation, transpiration, condensation, precipitation, and runoff
Critical Formulas and Calculations
Energy Balance Equation:
Incoming Solar Radiation = Reflected Radiation + Absorbed Radiation
1360 W/m² = (Albedo × 1360 W/m²) + Absorbed Energy
Net Primary Productivity (NPP):
NPP = GPP – Respiration
Where GPP is Gross Primary Productivity
10% Rule for Energy Transfer:
Energy available at each trophic level = 10% × Energy from previous level
Study Tips and Memory Aids
- Remember the “Four Spheres” with HBAG: Hydrosphere, Biosphere, Atmosphere, Geosphere
- Carbon Cycle Memory Device: “Plants Breathe, Burn, and Bury Carbon” (Photosynthesis, Respiration, Combustion, Sequestration)
- Nitrogen Cycle Steps: “Fix, Nitri, Assimilate, Deni” (Fixation, Nitrification, Assimilation, Denitrification)
- Soil Horizons: “Oh, A Boy Can Dig” (O, A, B, C, bedrock)
Exam Preparation Strategies
- Practice System Thinking: Always consider how changes in one sphere affect others
- Focus on Human Impacts: The AP exam emphasizes anthropogenic changes to natural systems
- Understand Feedback Loops: Know the difference between positive and negative feedbacks
- Master the Cycles: Be able to draw and explain all major biogeochemical cycles
- Connect Local to Global: Understand how local environmental issues relate to global patterns
Practice Questions
Multiple Choice Questions
- Which of the following best describes the relationship between Earth’s four major systems?
a) They operate independently with minimal interaction
b) They are interconnected with matter and energy flowing between them
c) Only the biosphere and atmosphere interact significantly
d) The geosphere controls all other systems
e) They only interact during extreme weather events - The process by which atmospheric nitrogen is converted into ammonia by bacteria is called:
a) Nitrification
b) Denitrification
c) Nitrogen fixation
d) Assimilation
e) Mineralization - Which soil horizon is characterized by the accumulation of materials leached from upper layers?
a) O horizon
b) A horizon
c) B horizon
d) C horizon
e) Bedrock - The primary cause of the enhanced greenhouse effect is:
a) Increased solar radiation
b) Volcanic eruptions
c) Human activities increasing greenhouse gas concentrations
d) Changes in Earth’s orbit
e) Decreased cloud cover - In the nitrogen cycle, the process that converts nitrates back to atmospheric nitrogen gas is:
a) Nitrogen fixation
b) Nitrification
c) Assimilation
d) Denitrification
e) Ammonification
Answer Key:
- b) They are interconnected with matter and energy flowing between them
- c) Nitrogen fixation
- c) B horizon
- c) Human activities increasing greenhouse gas concentrations
- d) Denitrification
Free Response Questions
Question 1: A farmer in the Midwest United States notices that crop yields have been declining despite consistent fertilizer application. Soil tests reveal that the soil pH has dropped from 7.0 to 5.5 over the past decade.
a) Explain how the change in soil pH could affect nutrient availability and plant growth. (3 points)
b) Describe TWO human activities that could contribute to soil acidification in agricultural systems. (2 points)
c) Propose TWO management strategies the farmer could implement to address this problem, and explain how each strategy would help restore soil pH. (4 points)
d) Explain how this local soil problem could potentially affect the broader nitrogen cycle in the region. (3 points)
Sample Answer Framework:
a) Lower pH reduces availability of essential nutrients (Ca, Mg, P) while increasing aluminum toxicity; affects enzyme function in plant roots; reduces beneficial soil microorganism activity
b) Acid rain from fossil fuel combustion; excessive use of nitrogen fertilizers that undergo nitrification
c) Lime application neutralizes acidity by adding calcium carbonate; cover cropping with legumes adds organic matter and reduces erosion
d) Acidified soils may reduce nitrogen-fixing bacteria populations; increased runoff could carry more nitrogen to water bodies; altered soil chemistry affects denitrification rates
FAQs:
Q: How do I remember all the different cycles for the AP exam?
A: Focus on understanding the key transformations rather than memorizing every detail. Create concept maps showing how each element moves between reservoirs, and practice drawing simplified versions of each cycle from memory.
Q: What’s the difference between weather and climate in terms of Earth systems?
A: Weather represents short-term atmospheric conditions (days to weeks), while climate represents long-term patterns (decades to centuries). Climate results from interactions between all four Earth systems, while weather primarily involves atmospheric processes.
Q: How do human activities affect natural cycles?
A: Humans accelerate some processes (like carbon release through fossil fuel burning), slow others (like carbon sequestration through deforestation), and create entirely new pathways (like industrial nitrogen fixation). The key is understanding that human activities often overwhelm natural rates of change.
Q: Why is the phosphorus cycle different from carbon and nitrogen cycles?
A: Phosphorus has no gaseous phase under normal Earth conditions, making it a purely sedimentary cycle. This means phosphorus pollution is more persistent, and phosphorus often becomes the limiting nutrient in ecosystems.
Q: How should I approach Earth systems questions on the AP exam?
A: Always think about connections between systems. Start by identifying which spheres are involved, then trace how matter or energy moves between them. Consider both natural processes and human impacts in your answers.
Conclusion and Further Exploration
Mastering AP Environmental Science Unit 4: Earth Systems and Resources provides you with the foundational understanding needed to comprehend virtually every environmental issue our planet faces. From climate change to resource depletion, from soil degradation to water pollution, all environmental challenges ultimately stem from disruptions in the delicate balance between Earth’s four major systems.
As you’ve learned throughout this comprehensive guide, the atmosphere, hydrosphere, geosphere, and biosphere don’t operate in isolation-they’re intimately connected through the continuous flow of matter and energy. Understanding these connections allows you to see environmental problems not as separate issues but as symptoms of larger system disruptions.
The biogeochemical cycles we’ve explored-carbon, nitrogen, phosphorus, and water-represent the planet’s recycling systems that have maintained conditions suitable for life for billions of years. Human activities are now altering these cycles at unprecedented rates, creating the environmental challenges that define our era.
Your knowledge of these Earth system interactions positions you to be part of the solution. Whether you pursue careers in environmental science, policy, education, or any other field, understanding how our planet works is increasingly valuable in a world facing complex environmental challenges.
Remember that environmental science is fundamentally about connections-between human activities and natural systems, between local actions and global consequences, between present decisions and future impacts. As you prepare for the AP exam and beyond, continue to think systemically about environmental issues.
The APES exam will test not just your knowledge of facts but your ability to analyze complex environmental scenarios, evaluate evidence, and propose science-based solutions. Use the practice questions and study strategies in this guide to develop these critical thinking skills.
Recommended Resources for Further Learning
- NASA Earth System Science: earthsystem.nasa.gov – Comprehensive resources on Earth system interactions with real-time data and satellite imagery
- USGS Water Resources: water.usgs.gov – Detailed information on water cycles, groundwater, and surface water interactions
- NOAA Climate.gov: climate.gov – Excellent explanations of climate systems, including the role of oceans and atmosphere
- Soil Science Society of America: soils.org – In-depth resources on soil formation, chemistry, and conservation
- Global Carbon Atlas: globalcarbonatlas.org – Interactive tools for exploring global carbon cycle data and trends
These resources will help you deepen your understanding of Earth systems and stay current with the latest research and discoveries. Remember, environmental science is a rapidly evolving field, and staying informed about new developments will enhance both your exam performance and your ability to address future environmental challenges.
Good luck with your AP Environmental Science studies, and remember that understanding Earth systems is the first step toward becoming an informed environmental steward!
Recommended –


1 thought on “AP Environmental Science Unit 4: Earth Systems and Resources – Complete Guide to Mastering the Exam”