The Electric World of Chemistry Around You
Picture this: You wake up to your smartphone alarm, powered by a lithium-ion battery. You brush your teeth with fluoride toothpaste, then grab a snack from your refrigerator. Later, you might notice rust forming on an old bicycle chain or watch silver jewelry tarnish over time. What connects all these everyday experiences? The fascinating world of electrochemistry!
Electrochemistry isn’t just another chapter in your textbook-it’s the science that powers our modern world. From the batteries in your devices to the galvanization that protects metal structures, from the electroplating that gives your jewelry its shine to the fuel cells that might power future cars, electrochemistry is everywhere. Even your own body runs on electrochemical processes-your nerve impulses are essentially electrical signals traveling through ion-conducting solutions!
As you embark on mastering CBSE Class 12 Chemistry Unit 2: Electrochemistry, you’re not just preparing for board exams. You’re unlocking the secrets behind technologies that define our daily lives. This comprehensive guide will transform what might seem like complex theories into clear, understandable concepts that you’ll actually enjoy learning.
Whether you’re aiming for that perfect score in your boards, preparing for competitive exams like JEE or NEET, or simply satisfying your curiosity about how the world works, this guide is your complete companion. We’ll journey together from the basic principles of oxidation and reduction to the sophisticated applications of electrochemical cells, ensuring you master every concept with confidence.
Learning Objectives: Your Roadmap to Electrochemistry Mastery
By the end of this comprehensive study guide, you will confidently:
- Master Redox Fundamentals: Understand oxidation and reduction processes, identify oxidizing and reducing agents, and balance complex redox equations using both oxidation number and half-reaction methods.
- Navigate Electrochemical Cells: Distinguish between galvanic and electrolytic cells, construct and interpret cell diagrams, and predict spontaneous reactions based on electrode potentials.
- Apply the Nernst Equation: Calculate cell potentials under non-standard conditions, understand concentration effects on EMF, and solve numerical problems involving equilibrium constants.
- Understand Conductance Phenomena: Analyze ionic conductivity, apply Kohlrausch’s law, and calculate degree of dissociation for weak electrolytes.
- Master Electrolysis Applications: Predict electrolysis products, perform quantitative electrolysis calculations using Faraday’s laws, and understand industrial electrochemical processes.
- Connect Theory to Applications: Relate electrochemical principles to real-world applications including batteries, fuel cells, corrosion prevention, and electroplating processes.
1. Redox Reactions: The Foundation of Electrochemistry
Understanding Oxidation and Reduction Beyond Memorization
You’ve probably memorized “OIL RIG” (Oxidation Is Loss, Reduction Is Gain) for electrons, but let’s dig deeper. Think of oxidation and reduction as a cosmic dance of electrons. When magnesium burns in air to form magnesium oxide, magnesium atoms literally give up electrons to oxygen atoms. The magnesium is being oxidized (losing electrons), while oxygen is being reduced (gaining electrons).
PROCESS: Complete Oxidation-Reduction Analysis
Let’s analyze the reaction: Zn + Cu²⁺ → Zn²⁺ + Cu
Step 1: Identify oxidation states
- Zn starts at 0, ends at +2 (loses 2 electrons – oxidized)
- Cu starts at +2, ends at 0 (gains 2 electrons – reduced)
Step 2: Write half-reactions
- Oxidation half: Zn → Zn²⁺ + 2e⁻
- Reduction half: Cu²⁺ + 2e⁻ → Cu
Step 3: Identify agents
- Zn is the reducing agent (causes Cu²⁺ to be reduced)
- Cu²⁺ is the oxidizing agent (causes Zn to be oxidized)
Balancing Redox Equations: Your Step-by-Step Toolkit
Method 1: Oxidation Number Method
Let’s balance: Cr₂O₇²⁻ + Fe²⁺ + H⁺ → Cr³⁺ + Fe³⁺ + H₂O
Step 1: Identify oxidation state changes
- Cr: +6 to +3 (decrease of 3, × 2 atoms = 6e⁻ gained)
- Fe: +2 to +3 (increase of 1, × ? atoms = ?e⁻ lost)
Step 2: Balance electron transfer
For 6e⁻ gained by Cr, we need 6e⁻ lost by Fe, so 6 Fe²⁺ ions
Step 3: Balance atoms and charge
Cr₂O₇²⁻ + 6Fe²⁺ + 14H⁺ → 2Cr³⁺ + 6Fe³⁺ + 7H₂O
Common Error Alert: Students often forget that dichromate contains 2 chromium atoms, leading to incorrect electron calculations. Always count atoms carefully!
Method 2: Half-Reaction Method (Ion-Electron Method)
This method is particularly powerful for complex reactions in acidic or basic solutions:
Step 1: Write separate half-reactions
Step 2: Balance atoms other than H and O
Step 3: Balance O by adding H₂O
Step 4: Balance H by adding H⁺ (acidic) or OH⁻ (basic)
Step 5: Balance charge by adding electrons
Step 6: Equalize electrons and add half-reactions
Chemistry Check: Can you identify the oxidizing agent in the reaction: KMnO₄ + HCl → KCl + MnCl₂ + Cl₂ + H₂O?
Answer: KMnO₄ (specifically, Mn⁷⁺ is reduced to Mn²⁺)
2. Electrochemical Cells: Where Chemistry Meets Electricity
Galvanic Cells: Nature’s Batteries
Imagine you could harness the energy released when zinc reacts with copper sulfate solution. That’s exactly what Alessandro Volta did in 1800, creating the first battery! A galvanic cell converts chemical energy into electrical energy through spontaneous redox reactions.
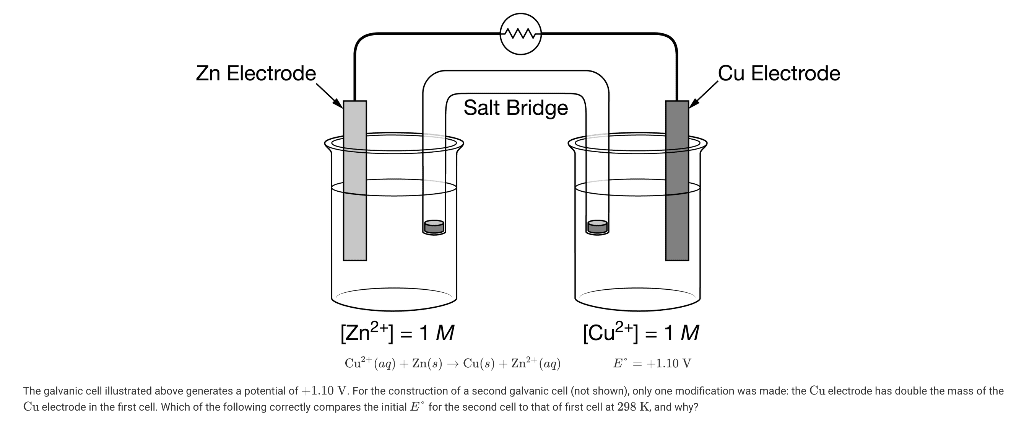
Key Components Explained:
- Anode (Negative Terminal): Where oxidation occurs
- Electrons are produced here
- Example: Zn → Zn²⁺ + 2e⁻
- Cathode (Positive Terminal): Where reduction occurs
- Electrons are consumed here
- Example: Cu²⁺ + 2e⁻ → Cu
- Salt Bridge: The unsung hero
- Maintains electrical neutrality
- Allows ion flow without mixing solutions
- Usually contains KCl or NH₄NO₃
Real-World Chemistry: Your car battery is essentially a lead-acid galvanic cell. The lead dioxide cathode and lead anode react with sulfuric acid to provide the electrical energy needed to start your engine!
Cell Notation: The Electrochemical Shorthand
Writing cell notation is like creating a chemical blueprint. For our zinc-copper cell:
Zn(s) | Zn²⁺(aq) || Cu²⁺(aq) | Cu(s)
Reading from left to right:
- Anode material | Anode solution || Cathode solution | Cathode material
- Single line (|) = phase boundary
- Double line (||) = salt bridge
Common Error Alert: Students often write cathode first. Remember: anode always comes first in cell notation, just like “A” comes before “C” in the alphabet!
Standard Electrode Potentials: The Electrochemical Scale
Think of electrode potentials like a ladder of reactivity. The Standard Hydrogen Electrode (SHE) sits at the middle (0.00 V), and all other electrodes are measured relative to it.
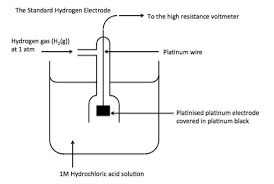
Understanding the Electrochemical Series:
- Higher E° values: Stronger oxidizing agents (more likely to be reduced)
- Lower E° values: Stronger reducing agents (more likely to be oxidized)
Process Analysis: Predicting Cell Reactions
- Identify the two half-reactions
- The half-reaction with higher E° occurs at cathode (reduction)
- The half-reaction with lower E° occurs at anode (oxidation)
- Calculate E°cell = E°cathode – E°anode
Example Calculation:
For Zn|Zn²⁺||Cu²⁺|Cu cell:
- E°(Cu²⁺/Cu) = +0.34 V
- E°(Zn²⁺/Zn) = -0.76 V
- E°cell = 0.34 – (-0.76) = 1.10 V
Since E°cell > 0, the reaction is spontaneous!
3. The Nernst Equation: Electrochemistry in the Real World
When Conditions Aren’t Standard
In the real world, concentrations aren’t always 1 M, temperatures aren’t always 25°C, and pressures aren’t always 1 atm. Enter the Nernst equation—your tool for calculating cell potentials under any conditions!
The Nernst Equation:
Ecell = E°cell – (RT/nF) ln Q
At 25°C, this simplifies to:
Ecell = E°cell – (0.0591/n) log Q
Where:
- R = 8.314 J/mol·K (gas constant)
- T = temperature in Kelvin
- n = number of electrons transferred
- F = 96,485 C/mol (Faraday constant)
- Q = reaction quotient
Historical Context: Walther Nernst won the Nobel Prize in Chemistry (1920) for his work on thermochemistry, including this fundamental equation that bridges thermodynamics and electrochemistry.
Practical Applications of the Nernst Equation
Example 1: Concentration Cell
Consider: Zn|Zn²⁺(0.01 M)||Zn²⁺(1.0 M)|Zn
Even though both electrodes are zinc, different concentrations create a potential difference!
Ecell = 0 – (0.0591/2) log(0.01/1.0) = 0.0591 V
Example 2: pH Measurement
The pH electrode in your chemistry lab works on Nernst equation principles:
E = E° – (0.0591) pH
Chemistry Check: If the concentration of Cu²⁺ in a Cu-Zn cell decreases, what happens to the cell potential?
Answer: The cell potential decreases because the driving force for the reaction decreases.
Equilibrium and the Nernst Equation
At equilibrium, Ecell = 0, and the Nernst equation becomes:
E°cell = (0.0591/n) log Keq
This beautiful relationship connects electrochemistry to chemical equilibrium!
Process Analysis: Calculating Equilibrium Constants
- Set up the balanced cell reaction
- Identify n (electrons transferred)
- Use E°cell = (0.0591/n) log Keq
- Solve for Keq
Example: For the Zn-Cu cell (E°cell = 1.10 V, n = 2):
1.10 = (0.0591/2) log Keq
log Keq = 37.2
Keq = 1.6 × 10³⁷
This huge equilibrium constant confirms that the reaction goes essentially to completion!
4. Conductance in Electrolytic Solutions
Understanding Ionic Conductivity
Unlike metals where electrons carry current, electrolytic solutions conduct through ion movement. Picture a crowded hallway where students (ions) move in opposite directions-cations toward the negative electrode, anions toward the positive electrode.
Key Concepts:
Conductance (G): The reciprocal of resistance
G = 1/R, measured in siemens (S)
Conductivity (κ): Conductance per unit length and area
κ = G × (l/A), measured in S/m
Molar Conductivity (Λm): Conductivity per mole of electrolyte
Λm = κ/C, where C is molar concentration
Factors Affecting Ionic Conductivity
1. Nature of Electrolyte
- Strong electrolytes: Complete dissociation, higher conductivity
- Weak electrolytes: Partial dissociation, lower conductivity
2. Concentration Effects
- Strong Electrolytes: Λm decreases with increasing concentration due to interionic attractions
- Weak Electrolytes: Λm increases with decreasing concentration due to increased dissociation
3. Temperature
Higher temperature increases ion mobility and conductivity
Real-World Chemistry: Water purity testing relies on conductivity measurements. Pure water has very low conductivity, while contaminated water conducts electricity due to dissolved ions.
Kohlrausch’s Law: The Independence Principle
Friedrich Kohlrausch discovered that at infinite dilution, each ion contributes independently to molar conductivity:
Λ°m = λ°+ + λ°-
Where λ° represents limiting ionic conductivities.
Applications of Kohlrausch’s Law:
1. Calculating Λ°m for Weak Electrolytes
For CH₃COOH:
Λ°m(CH₃COOH) = λ°(CH₃COO⁻) + λ°(H⁺)
2. Determining Degree of Dissociation
α = Λm/Λ°m
3. Finding Dissociation Constant
For weak acid: Ka = (Cα²)/(1-α)
Process Analysis: Complete Weak Acid Analysis
- Measure conductivity of weak acid solution
- Calculate molar conductivity (Λm)
- Find Λ°m using Kohlrausch’s law
- Calculate degree of dissociation (α)
- Determine dissociation constant (Ka)
Example Calculation:
For 0.01 M CH₃COOH with Λm = 16.2 S cm²/mol:
Given: Λ°m = 390.5 S cm²/mol
α = 16.2/390.5 = 0.0415
Ka = (0.01 × 0.0415²)/(1-0.0415) = 1.8 × 10⁻⁵
5. Electrolysis: Forcing Non-Spontaneous Reactions
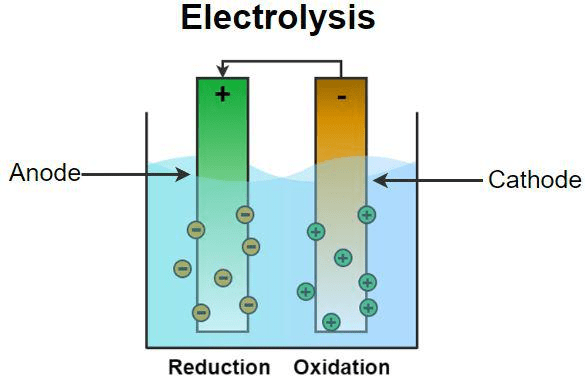
Electrolytic Cells vs. Galvanic Cells
If galvanic cells are like water flowing downhill (spontaneous), electrolytic cells are like pumping water uphill (non-spontaneous, requiring external energy). An external power source forces electrons to flow in the opposite direction to their natural tendency.
Key Differences:
| Aspect | Galvanic Cell | Electrolytic Cell |
|---|---|---|
| Energy | Chemical → Electrical | Electrical → Chemical |
| ΔG | Negative (spontaneous) | Positive (non-spontaneous) |
| Cathode | Positive terminal | Negative terminal |
| Anode | Negative terminal | Positive terminal |
Common Error Alert: Students often confuse electrode signs between galvanic and electrolytic cells. Remember: In electrolytic cells, the cathode connects to the negative terminal of the external battery!
Predicting Electrolysis Products
The key is understanding that the most easily reduced species goes to the cathode, while the most easily oxidized species goes to the anode.
At the Cathode (Reduction):
Order of preference: Metal ions > H⁺ (from water) > H₂O
- If metal is above hydrogen in electrochemical series: H₂ evolves
- If metal is below hydrogen: Metal deposits
At the Anode (Oxidation):
Order of preference: Anions (except F⁻) > OH⁻ (from water) > H₂O
- Halides (except F⁻): Halogen gas evolves
- Oxyanions (SO₄²⁻, NO₃⁻): O₂ evolves
- OH⁻: O₂ evolves
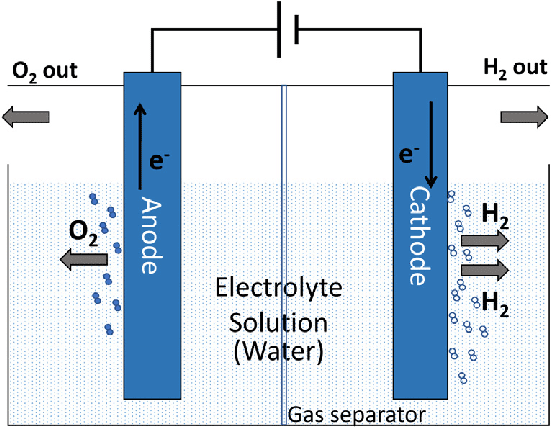
Process Analysis: Electrolysis of Aqueous NaCl
- Possible cathode reactions:
- Na⁺ + e⁻ → Na (E° = -2.71 V)
- 2H₂O + 2e⁻ → H₂ + 2OH⁻ (E° = -0.83 V)
- Water reduction occurs (higher E°) → H₂ gas
- Possible anode reactions:
- 2Cl⁻ → Cl₂ + 2e⁻ (E° = -1.36 V)
- 2H₂O → O₂ + 4H⁺ + 4e⁻ (E° = -1.23 V)
- Chloride oxidation occurs (lower magnitude) → Cl₂ gas
Overall: 2NaCl + 2H₂O → H₂ + Cl₂ + 2NaOH
This is how industrial chlorine and sodium hydroxide are produced!
Faraday’s Laws of Electrolysis
Michael Faraday’s laws quantify the relationship between electricity and chemical change:
First Law: The amount of substance deposited is directly proportional to the quantity of electricity passed.
m = ZIt
Second Law: When the same quantity of electricity passes through different electrolytes, the amounts deposited are proportional to their equivalent weights.
m = (M/nF) × It
Where:
- m = mass deposited (g)
- M = molar mass (g/mol)
- n = number of electrons
- F = 96,485 C/mol
- I = current (A)
- t = time (s)
Practical Calculation Example:
How long does it take to deposit 2.54 g of copper from CuSO₄ using 2 A current?
Given: M(Cu) = 63.5 g/mol, n = 2
2.54 = (63.5/2×96485) × 2 × t
t = 1930 s = 32.2 minutes
Current Research: Modern electroplating uses pulsed current instead of steady DC to achieve better surface finishes and reduce hydrogen evolution-a principle used in manufacturing computer chips and jewelry!
6. Batteries and Fuel Cells: Portable Power Sources
Primary Batteries: Use Once, Dispose
Dry Cell (Leclanche Cell)
The common flashlight battery you know:
- Anode: Zn container
- Cathode: Carbon rod surrounded by MnO₂
- Electrolyte: NH₄Cl paste
Anode reaction: Zn → Zn²⁺ + 2e⁻
Cathode reaction: 2MnO₂ + 2NH₄⁺ + 2e⁻ → Mn₂O₃ + H₂O + 2NH₃
Cell voltage: ~1.5 V
Alkaline Battery
Better performance through alkaline electrolyte:
- Anode: Zn powder
- Cathode: MnO₂
- Electrolyte: KOH
Advantages: Longer life, better performance at low temperatures, less leakage
Secondary Batteries: Rechargeable Power
Lead-Acid Battery
The workhorse of automotive industry:
Discharge:
- Anode: Pb + SO₄²⁻ → PbSO₄ + 2e⁻
- Cathode: PbO₂ + SO₄²⁻ + 4H⁺ + 2e⁻ → PbSO₄ + 2H₂O
- Cell voltage: ~2 V (12 V battery has 6 cells)
Charge: Reverse the above reactions using external power
Real-World Chemistry: Why does your car battery die in winter? Cold temperatures reduce ion mobility in the sulfuric acid electrolyte, decreasing battery performance!
Lithium-Ion Battery
The power behind modern electronics:
- Anode: Graphite (Li intercalated)
- Cathode: LiCoO₂ or similar
- Electrolyte: Organic solvent with Li⁺ salts
Discharge: Li → Li⁺ + e⁻ (at anode)
Charge: External power drives Li⁺ back to anode
Advantages: High energy density, low self-discharge, no memory effect
Fuel Cells: The Future of Clean Energy
Fuel cells convert chemical energy directly to electrical energy with high efficiency and minimal pollution.
Hydrogen Fuel Cell:
- Anode: H₂ → 2H⁺ + 2e⁻
- Cathode: O₂ + 4H⁺ + 4e⁻ → 2H₂O
- Overall: 2H₂ + O₂ → 2H₂O
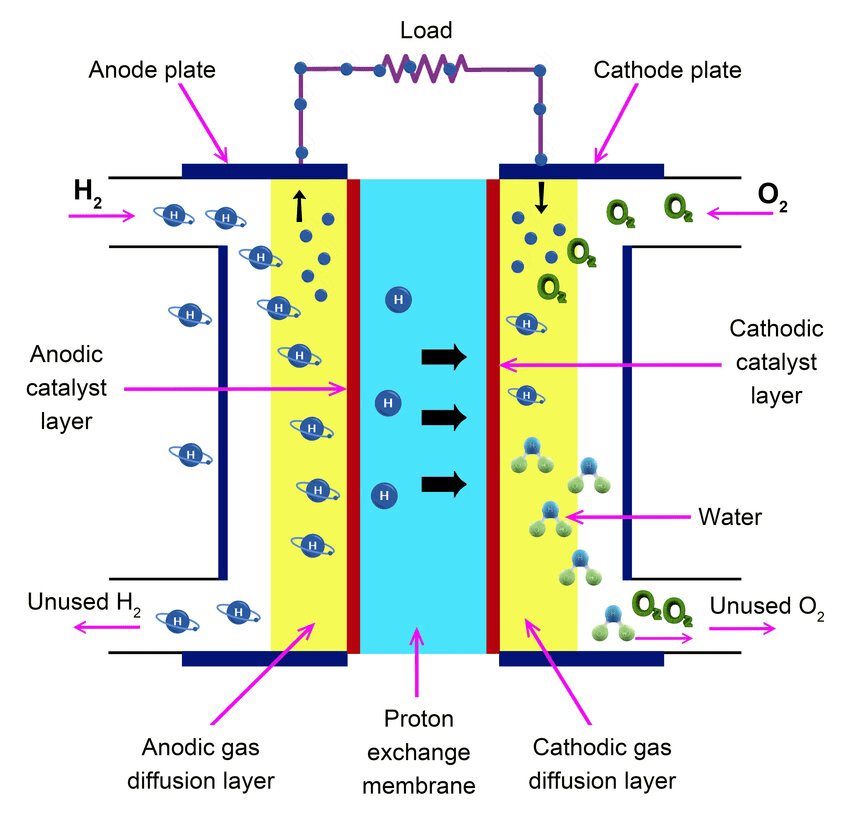
Environmental Advantage: Only byproduct is water! NASA has used fuel cells in space missions since the 1960s.
Current Research: Scientists are developing fuel cells that use methanol, ethanol, and even glucose as fuels, potentially powering devices using biological molecules!
7. Corrosion: When Electrochemistry Goes Wrong
Understanding the Corrosion Process
Corrosion is essentially unwanted electrochemical reaction. When iron rusts, different parts of the metal surface act as anodes and cathodes, creating tiny galvanic cells.
Rusting of Iron: A Complex Electrochemical Process
Step 1: Initial oxidation
Anode: Fe → Fe²⁺ + 2e⁻
Step 2: Oxygen reduction
Cathode: O₂ + 4H⁺ + 4e⁻ → 2H₂O (acidic conditions)
Or: O₂ + 2H₂O + 4e⁻ → 4OH⁻ (neutral/basic conditions)
Step 3: Further oxidation
Fe²⁺ + O₂ + H₂O → Fe₂O₃·xH₂O (rust)
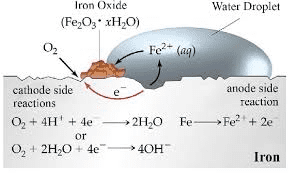
Factors Accelerating Corrosion:
- Moisture: Provides electrolyte medium
- Oxygen: Essential for cathodic reaction
- Acids: Lower pH increases H⁺ concentration
- Salt: Increases conductivity of electrolyte
- Impurities: Create galvanic cells
Real-World Chemistry: Why do cars rust faster in coastal areas? Salt spray increases the electrolyte conductivity, accelerating the electrochemical corrosion process!
Corrosion Prevention Methods
1. Barrier Protection
- Painting, oiling, greasing
- Prevents oxygen and moisture contact
2. Galvanization
- Coating iron with zinc
- Zinc acts as sacrificial anode (E°Zn < E°Fe)
3. Cathodic Protection
- Connect iron to more active metal (Mg, Zn)
- Active metal corrodes instead of iron
4. Alloying
- Stainless steel contains chromium
- Forms protective Cr₂O₃ layer
Process Analysis: Sacrificial Protection
- More active metal becomes anode
- Iron becomes cathode (protected)
- Active metal gradually corrodes away
- Replace sacrificial metal periodically
Historical Context: The Statue of Liberty’s green color isn’t paint—it’s patina! Copper reacts with air to form Cu₂CO₃(OH)₂, which actually protects the underlying metal from further corrosion.
8. Industrial Applications of Electrochemistry
Electroplating: Beauty and Protection
Electroplating deposits a thin layer of one metal onto another using electrolysis. It’s both decorative and protective!
Silver Plating Process:
- Object to be plated: Connected to cathode
- Silver electrode: Connected to anode
- Electrolyte: AgNO₃ solution
Reactions:
- Anode: Ag → Ag⁺ + e⁻
- Cathode: Ag⁺ + e⁻ → Ag (deposits on object)
Process Variables:
- Current density: Controls plating rate and quality
- Temperature: Affects crystal structure
- pH: Influences deposit properties
- Additives: Improve brightness and smoothness
Quality Control: Uniform plating requires proper surface preparation, controlled current distribution, and optimized electrolyte composition.
Electrorefining: Purifying Metals
Copper refining produces 99.99% pure copper from impure metal:
Setup:
- Anode: Impure copper
- Cathode: Pure copper sheet
- Electrolyte: CuSO₄ solution
Process:
- Cu from impure anode dissolves: Cu → Cu²⁺ + 2e⁻
- Cu²⁺ deposits on pure cathode: Cu²⁺ + 2e⁻ → Cu
- Impurities either don’t dissolve or don’t deposit
Economic Impact: This process produces millions of tons of pure copper annually for electrical wiring and electronics!
Electrochemical Manufacturing
Aluminum Production (Hall-Héroult Process):
Raw material: Al₂O₃ (alumina) dissolved in molten cryolite
- Cathode: Carbon-lined steel pot
- Anode: Carbon electrodes
- Temperature: ~960°C
Reactions:
- Cathode: Al³⁺ + 3e⁻ → Al
- Anode: 2O²⁻ → O₂ + 4e⁻
Energy Requirement: Enormous! It takes about 13-15 kWh to produce 1 kg of aluminum, which is why aluminum recycling is so important environmentally.
Chlor-Alkali Industry:
Electrolysis of brine produces three valuable chemicals:
- Chlorine: For PVC, bleach, pharmaceuticals
- Sodium hydroxide: For soap, paper, textiles
- Hydrogen: For ammonia synthesis, fuel
Current Research: Scientists are developing more energy-efficient electrolysis processes and exploring electrochemical methods for carbon dioxide reduction-potentially turning CO₂ into useful chemicals!
9. Advanced Problem-Solving Strategies
Mastering Numerical Problems
Strategy 1: Systematic Approach
- Identify what’s given and what’s asked
- Choose appropriate equation/concept
- Substitute values carefully
- Calculate step by step
- Check units and reasonableness
Strategy 2: Common Problem Types
Type 1: Cell Potential Calculations
Given: E°values, concentrations
Find: Ecell under specific conditions
Example: Calculate Ecell for Zn|Zn²⁺(0.1M)||Ag⁺(0.01M)|Ag
E°(Zn²⁺/Zn) = -0.76 V, E°(Ag⁺/Ag) = +0.80 V
Solution:
- E°cell = 0.80 – (-0.76) = 1.56 V
- Reaction: Zn + 2Ag⁺ → Zn²⁺ + 2Ag (n = 2)
- Q = [Zn²⁺]/[Ag⁺]² = 0.1/(0.01)² = 1000
- Ecell = 1.56 – (0.0591/2)log(1000) = 1.56 – 0.089 = 1.47 V
Type 2: Electrolysis Calculations
Given: Current, time, electrolyte
Find: Mass deposited or gas evolved
Example: How much Cu deposits in 2 hours using 5 A current in CuSO₄?
Solution:
- Q = It = 5 × 2 × 3600 = 36,000 C
- Moles of e⁻ = 36,000/96,485 = 0.373 mol
- Cu²⁺ + 2e⁻ → Cu, so moles Cu = 0.373/2 = 0.187 mol
- Mass Cu = 0.187 × 63.5 = 11.9 g
Chemistry Check: In the above problem, what volume of O₂ at STP would evolve at the anode?
Answer: 0.187/2 = 0.094 mol O₂ = 0.094 × 22.4 = 2.1 L
Tackling Conceptual Questions
Strategy: Build connections between concepts
Example Question: “Why does conductivity of weak acids increase upon dilution while that of strong acids decreases?”
Answer Framework:
- Define the phenomenon clearly
- Explain underlying principles (degree of dissociation vs. ion concentration)
- Use equations where relevant (α = Λm/Λ°m)
- Connect to real examples
Avoiding Common Pitfalls
Error 1: Confusing standard conditions
- Standard conditions: 1 M, 25°C, 1 atm
- STP: 0°C, 1 atm (different from standard!)
Error 2: Wrong electrode identification
- Remember: Reduction happens at cathode, oxidation at anode
- In galvanic cells: Cathode is positive, anode is negative
- In electrolytic cells: Cathode is negative, anode is positive
Error 3: Incorrect Nernst equation application
- Use log₁₀, not ln in the 0.0591 version
- Reaction quotient Q has products over reactants
- Include all species in their proper stoichiometric ratios
10. Exam Preparation and Success Strategies
Understanding the CBSE Pattern
Question Types and Weightage:
- 1-mark questions: Definitions, simple calculations
- 2-mark questions: Short numerical problems, explanations
- 3-mark questions: Medium-length calculations, processes
- 5-mark questions: Complex problems, detailed explanations
Frequently Tested Topics:
- Cell potential calculations (Nernst equation)
- Electrolysis problems (Faraday’s laws)
- Conductivity (Kohlrausch’s law applications)
- Corrosion and prevention
- Battery comparisons
Time Management During Exams
The 4-3-2-1 Strategy:
- 4 minutes: Read question carefully, identify what’s asked
- 3 minutes: Plan approach, list given data
- 2 minutes: Solve step by step
- 1 minute: Check answer, units, significant figures
Common Time Wasters:
- Spending too long on unfamiliar problems
- Not showing proper working
- Forgetting to mention units
- Second-guessing correct answers
Last-Minute Revision Checklist
Formulas to Memorize:
- Nernst equation: Ecell = E°cell – (0.0591/n)logQ
- Relationship: ΔG° = -nFE°cell
- Conductivity: κ = G × (l/A)
- Kohlrausch’s law: Λ°m = λ°+ + λ°-
- Faraday’s law: m = (M/nF) × It
Key Relationships:
- α = Λm/Λ°m (degree of dissociation)
- Ka = Cα²/(1-α) (for weak acids)
- log Keq = nE°cell/0.0591
Important Constants:
- F = 96,485 C/mol
- R = 8.314 J/mol·K
- At 25°C: RT/F = 0.0257 V
Answer Writing Techniques
For Numerical Problems:
- Write given data clearly
- State the formula you’ll use
- Show substitution step by step
- Include units in final answer
- Box the final answer
For Theory Questions:
- Start with definition if relevant
- Use bullet points for clarity
- Include diagrams where asked
- Give examples to illustrate points
- Conclude with significance or applications
Sample Answer Format:
Question: Explain the working of a fuel cell.
Answer:
Definition: A fuel cell is an electrochemical device that converts chemical energy directly into electrical energy.
Working Principle:
- Anode reaction: H₂ → 2H⁺ + 2e⁻
- Cathode reaction: ½O₂ + 2H⁺ + 2e⁻ → H₂O
- Overall reaction: H₂ + ½O₂ → H₂O
Advantages:
- High efficiency (~80%)
- Environmentally friendly
- Quiet operation
- Continuous operation with fuel supply
Practice Problems with Detailed Solutions
Multiple Choice Questions (MCQs)
Q1. The standard electrode potential of the half-cell reactions are:
Zn²⁺ + 2e⁻ → Zn; E° = -0.76 V
Fe²⁺ + 2e⁻ → Fe; E° = -0.44 V
The EMF of the cell reaction Fe²⁺ + Zn → Zn²⁺ + Fe is:
(a) -0.32 V (b) +0.32 V (c) -1.20 V (d) +1.20 V
Solution:
Step 1: Identify cathode and anode
Since Fe²⁺/Fe has higher E° value, it will be cathode (reduction)
Zn/Zn²⁺ will be anode (oxidation)
Step 2: Calculate EMF
E°cell = E°cathode – E°anode = -0.44 – (-0.76) = +0.32 V
Answer: (b) +0.32 V
Q2. The molar conductivity of 0.025 M methanoic acid is 46.1 S cm²/mol and its degree of dissociation is 0.023. The limiting molar conductivity of methanoic acid is:
(a) 1000 S cm²/mol (b) 2000 S cm²/mol (c) 4000 S cm²/mol (d) 3000 S cm²/mol
Solution:
Using: α = Λm/Λ°m
0.023 = 46.1/Λ°m
Λ°m = 46.1/0.023 = 2000 S cm²/mol
Answer: (b) 2000 S cm²/mol
Numerical Problems
Q3. Calculate the standard cell potential of galvanic cell in which the following reaction takes place:
Fe²⁺(aq) + Ag⁺(aq) → Fe³⁺(aq) + Ag(s)
Calculate the equilibrium constant of the reaction.
[Given: E°(Fe³⁺/Fe²⁺) = 0.77 V; E°(Ag⁺/Ag) = 0.80 V]
Solution:
Step 1: Balance the equation
Fe²⁺(aq) + Ag⁺(aq) → Fe³⁺(aq) + Ag(s)
Step 2: Identify half-reactions
Reduction (cathode): Ag⁺ + e⁻ → Ag; E° = 0.80 V
Oxidation (anode): Fe²⁺ → Fe³⁺ + e⁻; E° = 0.77 V
Step 3: Calculate standard cell potential
E°cell = E°cathode – E°anode = 0.80 – 0.77 = 0.03 V
Step 4: Calculate equilibrium constant
log Keq = nE°cell/0.0591 = 1 × 0.03/0.0591 = 0.51
Keq = 10^0.51 = 3.2
Answer: E°cell = 0.03 V; Keq = 3.2
Q4. A current of 1.5 A is passed through aqueous solution of CuSO₄ for 20 minutes. Calculate the mass of copper deposited at cathode.
[Given: Atomic mass of Cu = 63.5 u; F = 96500 C/mol]
Solution:
Step 1: Calculate total charge passed
Q = I × t = 1.5 A × 20 × 60 s = 1800 C
Step 2: Write electrode reaction
Cu²⁺ + 2e⁻ → Cu
Step 3: Apply Faraday’s law
Number of moles of electrons = Q/F = 1800/96500 = 0.0186 mol
Step 4: Calculate moles of Cu deposited
From stoichiometry: 2 moles e⁻ deposit 1 mole Cu
Moles of Cu = 0.0186/2 = 0.0093 mol
Step 5: Calculate mass
Mass of Cu = 0.0093 × 63.5 = 0.59 g
Answer: 0.59 g copper will be deposited.
Case Study Questions
Q5. Electrochemical cells are widely used in various applications. Consider a galvanic cell made up of Zn-electrode in ZnSO₄ solution and Ag-electrode in AgNO₃ solution.
Part A: Write the cell notation for this galvanic cell.
Part B: Which electrode will act as anode and which as cathode?
Part C: Calculate the standard EMF of the cell.
Part D: What will happen to the cell potential if concentration of Zn²⁺ is increased?
[Given: E°(Zn²⁺/Zn) = -0.76 V; E°(Ag⁺/Ag) = 0.80 V]
Solution:
Part A: Cell notation
Zn(s)|Zn²⁺(aq)||Ag⁺(aq)|Ag(s)
Part B: Electrode identification
Since E°(Ag⁺/Ag) > E°(Zn²⁺/Zn):
- Anode: Zn electrode (oxidation occurs)
- Cathode: Ag electrode (reduction occurs)
Part C: Standard EMF calculation
E°cell = E°cathode – E°anode = 0.80 – (-0.76) = 1.56 V
Part D: Effect of increasing [Zn²⁺]
Cell reaction: Zn + 2Ag⁺ → Zn²⁺ + 2Ag
Q = [Zn²⁺]/[Ag⁺]²
If [Zn²⁺] increases, Q increases.
From Nernst equation: Ecell = E°cell – (0.0591/n)logQ
As Q increases, logQ increases, so Ecell decreases.
Answer: The cell potential will decrease when [Zn²⁺] is increased.
Reasoning-Based Questions
Q6. Why is it not possible to measure the absolute electrode potential of a single electrode?
Solution:
It is impossible to measure the absolute electrode potential of a single electrode because:
Reason 1: No complete circuit
- A potential difference requires two points
- Single electrode cannot form a complete electrical circuit
- Voltmeter needs two terminals to measure potential difference
Reason 2: Thermodynamic limitation
- Absolute potential would require measurement against vacuum
- This is not practically achievable in electrochemical systems
- All measurements are relative to some reference point
Reason 3: Convention necessity
- Standard Hydrogen Electrode (SHE) is chosen as reference (E° = 0.00 V)
- All other electrode potentials are measured relative to SHE
- This provides a consistent, reproducible scale
Practical implication: We always measure cell potentials (difference between two electrodes) rather than individual electrode potentials.
Conclusion: Mastering Electrochemistry for Board Success
Congratulations! You’ve now journeyed through the fascinating world of electrochemistry, from the basic principles of redox reactions to the sophisticated applications powering our modern world. This isn’t just another chapter you’ve studied-you’ve gained insights into the fundamental processes that make batteries work, metals rust, and industrial electrochemical processes possible.
Key Takeaways for Board Exam Excellence
Conceptual Mastery: You now understand that electrochemistry is fundamentally about electron transfer. Whether it’s the spontaneous reactions in galvanic cells or the forced reactions in electrolytic cells, the common thread is the movement of electrons from reducing agents to oxidizing agents. This understanding will help you tackle any electrochemistry problem with confidence.
Mathematical Proficiency: The Nernst equation, Faraday’s laws, and Kohlrausch’s law are not just formulas to memorize-they’re tools that connect theoretical principles to real-world applications. Your ability to use these equations correctly will set you apart in numerical problems.
Application Awareness: From the lithium-ion battery in your smartphone to the galvanization protecting steel structures, electrochemistry is everywhere. This real-world perspective will help you appreciate the subject beyond exam requirements and potentially inspire future career choices.
Strategic Exam Approach
Time Allocation: Remember the 40-60 rule-spend 40% of your time understanding the problem and planning your approach, and 60% executing the solution. This prevents the common mistake of rushing into calculations without proper setup.
Error Prevention: Watch out for the most common mistakes:
- Confusing galvanic and electrolytic cell polarities
- Misapplying the Nernst equation
- Forgetting to balance redox equations properly
- Incorrect electrode identification in electrolysis
Answer Presentation: Structure your solutions clearly with proper headings, show all working steps, include units in final answers, and box your final results. Examiners appreciate organized presentations.
Beyond the Boards: Future Connections
The principles you’ve learned here form the foundation for advanced topics in physical chemistry, materials science, and environmental engineering. If you’re planning to pursue JEE or NEET, this solid understanding of electrochemistry will give you a significant advantage in tackling more complex problems involving thermodynamics and chemical equilibrium.
For those interested in engineering, remember that electrochemistry drives innovations in energy storage, corrosion prevention, and electrochemical manufacturing. The green energy revolution-from electric vehicles to renewable energy storage-depends heavily on electrochemical principles.
Final Revision Strategy
Week 1: Focus on fundamental concepts and basic calculations
- Redox reactions and balancing
- Cell potential calculations using standard electrode potentials
- Basic electrolysis problems
Week 2: Advance to complex applications
- Nernst equation problems under non-standard conditions
- Conductivity and Kohlrausch’s law applications
- Industrial processes and corrosion
Day Before Exam:
- Review formula sheet and important constants
- Practice writing balanced chemical equations
- Solve previous year questions for pattern recognition
Remember, electrochemistry rewards understanding over memorization. If you truly grasp why reactions occur, how electrons flow, and what drives electrochemical processes, you’ll find that even unfamiliar problems become solvable puzzles rather than insurmountable obstacles.
Your journey through CBSE Class 12 Chemistry Unit 2: Electrochemistry is now complete, but your exploration of this fascinating field has just begun. Carry forward this knowledge with confidence, knowing that you possess both the theoretical understanding and practical problem-solving skills to excel in your board exams and beyond.
Good luck with your examinations! The principles of electrochemistry will serve you well, not just in your exams, but in understanding the increasingly electrochemical world around us.
This comprehensive study guide provides all the theoretical knowledge, practical applications, and exam strategies needed for complete mastery of CBSE Class 12 Chemistry Unit 2: Electrochemistry. Regular practice with the included problems and consistent review of the key concepts will ensure excellent performance in board examinations.
Recommended –


1 thought on “CBSE Class 12 Chemistry Unit 2: Electrochemistry – Complete Master Guide for Board Exam Success”