The Amazing World of Enzymes
Imagine trying to light a campfire without matches or a lighter – it would be nearly impossible! Now picture your body trying to carry out thousands of chemical reactions without enzymes. Just like matches help start a fire, enzymes are the biological “matches” that make life possible by speeding up essential chemical reactions in your body.
Right now, as you’re reading this, millions of enzymes are working tirelessly in your cells – breaking down the food you ate for lunch, helping your muscles contract as you breathe, and even assisting in the complex process of thinking! From the moment you wake up until you fall asleep, enzymes are your body’s hardworking molecular machines.
This comprehensive guide will take you through everything you need to know about enzymes for your IGCSE Biology exam, explained in simple terms with plenty of examples and exam tips along the way.
What Are Enzymes? The Biological Catalysts
Definition and Basic Properties
Enzymes are biological catalysts made of protein that speed up chemical reactions in living organisms without being used up in the process.
Think of enzymes as extremely efficient workers in a factory. Just as a skilled worker can assemble products much faster than an untrained person, enzymes can speed up reactions that would otherwise take thousands of years to complete naturally – making them happen in milliseconds instead!
Key Characteristics of Enzymes:
- Protein structure – Made up of long chains of amino acids folded into specific 3D shapes
- Highly specific – Each enzyme typically works on one type of substrate (the substance it acts upon)
- Reusable – Not consumed in the reaction, so can be used repeatedly
- Lower activation energy – Reduce the energy needed to start a reaction
- Affected by conditions – Temperature, pH, and concentration influence their activity
Real-Life Examples of Enzymes in Action:
- Amylase in your saliva – Starts breaking down starchy foods like bread the moment you put them in your mouth
- Pepsin in your stomach – Breaks down proteins in your food using the acidic environment
- Catalase in your liver – Breaks down harmful hydrogen peroxide into harmless water and oxygen
- DNA polymerase – Helps copy DNA during cell division
The Structure of Enzymes: Understanding the Molecular Architecture
Primary to Quaternary Structure
Enzymes, being proteins, have a complex hierarchical structure that determines their function:
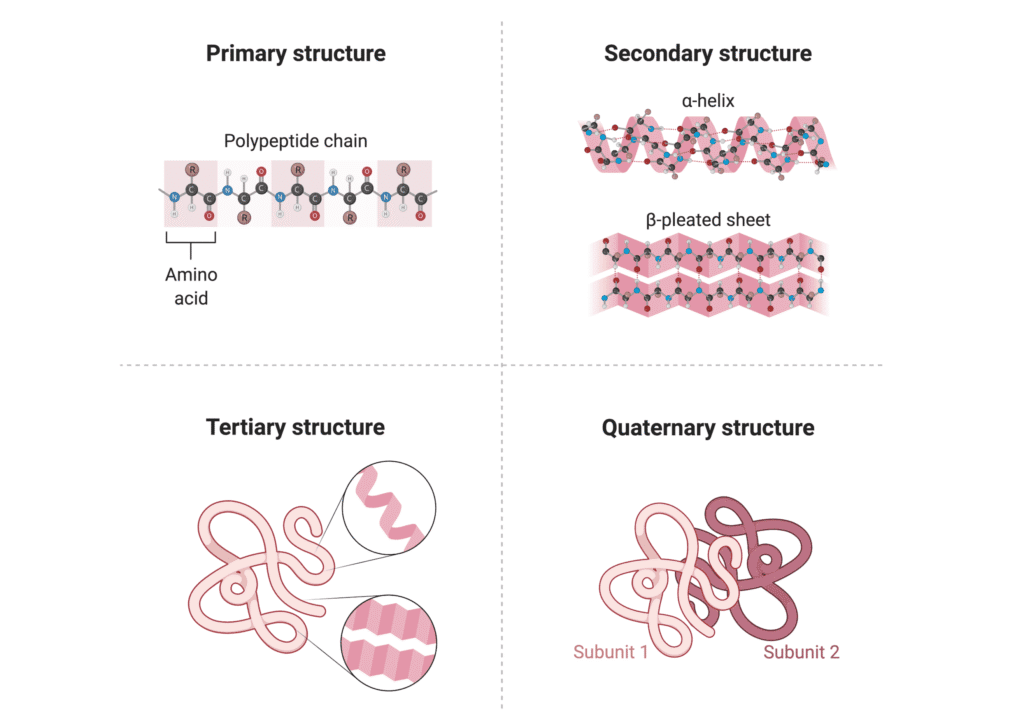
The Active Site: Where the Magic Happens
The active site is the most crucial part of an enzyme – it’s the specific region where the substrate binds and the reaction occurs. Think of it as a specialized workspace designed for one particular job.
Key features of the active site:
- Unique shape – Perfectly complementary to its specific substrate
- Chemical environment – Contains amino acid residues that facilitate the reaction
- Flexible structure – Can slightly change shape to accommodate the substrate
Enzyme-Substrate Complex Formation
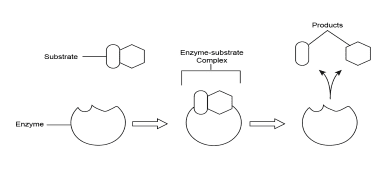
How Enzymes Work: The Lock and Key vs. Induced Fit Models
The Lock and Key Model
Proposed by Emil Fischer in 1894, this model suggests that:
- The enzyme (lock) has a rigid, fixed shape
- The substrate (key) fits perfectly into the active site
- Only the correct substrate can bind, like a specific key fitting a particular lock
Advantages: Simple to understand and visualize
Limitations: Doesn’t explain why enzymes can work with slightly different substrates
The Induced Fit Model (Current Understanding)
Developed by Daniel Koshland in 1958, this more accurate model proposes that:
- The enzyme is flexible and changes shape slightly when the substrate approaches
- The substrate also changes shape during binding
- This mutual adjustment creates the perfect fit for the reaction to occur
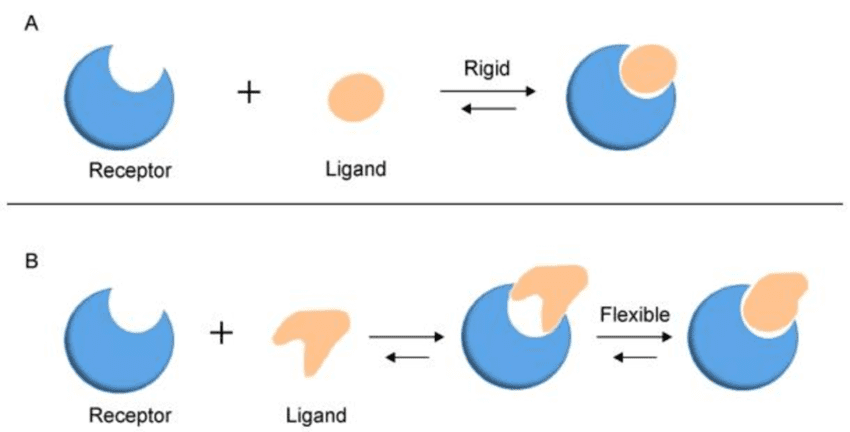
Why the Induced Fit Model is Better:
- Explains enzyme flexibility – Accounts for slight substrate variations
- Better reaction facilitation – The shape change helps stabilize the transition state
- Supported by evidence – X-ray crystallography studies confirm conformational changes
Factors Affecting Enzyme Activity
Understanding what influences enzyme activity is crucial for your IGCSE exam. Let’s explore each factor in detail:
1. Temperature Effects
The Relationship:
- Low temperatures – Enzymes work slowly due to reduced molecular movement
- Optimal temperature – Maximum enzyme activity (usually around 37°C for human enzymes)
- High temperatures – Enzyme denaturation and loss of function
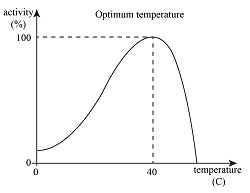
Memory Tip: Think of enzymes like Goldilocks – they need conditions that are “just right,” not too hot, not too cold!
2. pH Effects
Different enzymes have different optimal pH ranges:
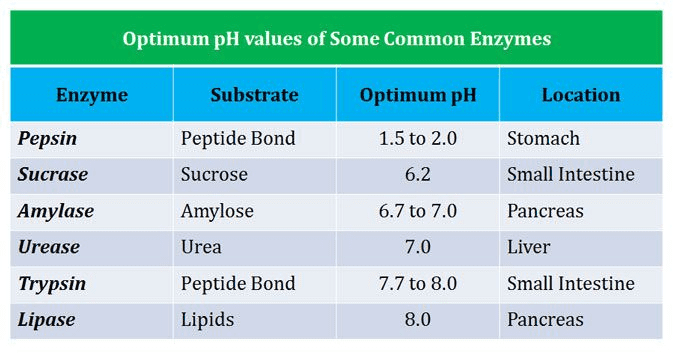
Why pH Matters:
- Changes in pH alter the enzyme’s shape by affecting ionic bonds
- Extreme pH can denature enzymes permanently
- Each enzyme evolved to work best in its natural environment
3. Enzyme Concentration
The Pattern:
- Increasing enzyme concentration initially increases reaction rate
- Plateau effect – Eventually, all substrate molecules are occupied
- Limiting factor shifts from enzyme to substrate availability
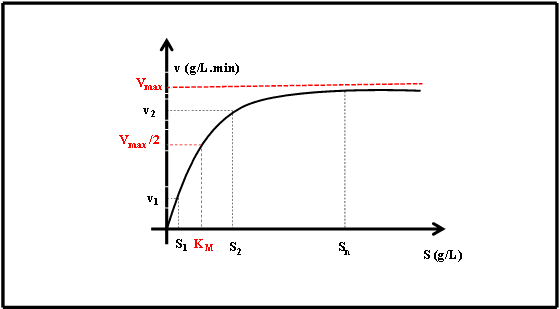
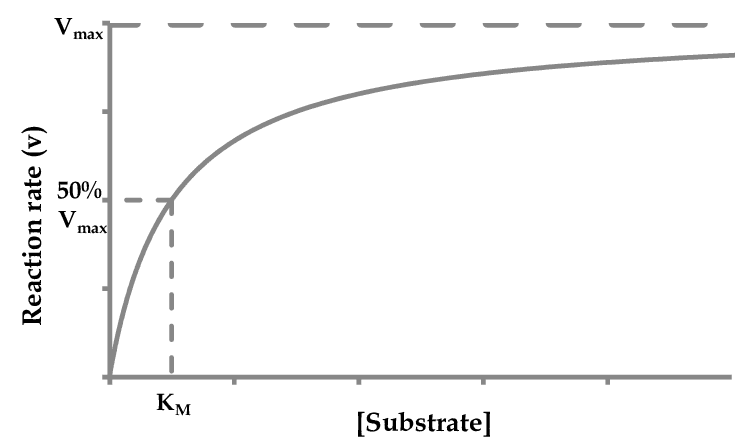
4. Substrate Concentration
The Relationship:
- Low substrate concentration – Reaction rate increases proportionally
- High substrate concentration – Enzyme saturation occurs
- Maximum velocity (Vmax) – Reached when all enzyme active sites are occupied
5. Presence of Inhibitors
Competitive Inhibition:
- Inhibitor competes with substrate for the active site
- Can be overcome by increasing substrate concentration
- Example: Statins competing with natural substrates in cholesterol synthesis
Non-competitive Inhibition:
- Inhibitor binds to a different site, changing enzyme shape
- Cannot be overcome by increasing substrate concentration
- Example: Heavy metals like mercury binding to enzyme sulfur groups
Important Enzyme Examples for IGCSE
Digestive Enzymes
1. Amylase
- Source: Salivary glands and pancreas
- Function: Breaks down starch into maltose
- Equation: Starch + Water → Maltose
- Optimal conditions: pH 6.7-7.0, 37°C
2. Pepsin
- Source: Stomach (gastric glands)
- Function: Breaks down proteins into smaller polypeptides
- Equation: Protein + Water → Polypeptides
- Optimal conditions: pH 1.5-2.0, 37°C
3. Lipase
- Source: Pancreas
- Function: Breaks down fats into fatty acids and glycerol
- Equation: Fat + Water → Fatty acids + Glycerol
- Optimal conditions: pH 8.0-8.5, 37°C
Metabolic Enzymes
4. Catalase
- Source: Most living cells (especially liver)
- Function: Breaks down toxic hydrogen peroxide
- Equation: 2H₂O₂ → 2H₂O + O₂
- Significance: Protective enzyme preventing cell damage
Practical Applications and Real-World Examples
Enzymes in Biotechnology
1. Food Industry:
- Pectinase – Clarifies fruit juices by breaking down pectin
- Lactase – Produces lactose-free milk for lactose-intolerant individuals
- Invertase – Converts sucrose to glucose and fructose in candy making
2. Medicine:
- Streptokinase – Dissolves blood clots in heart attack patients
- Penicillinase – Used to study antibiotic resistance
- Restriction enzymes – Cut DNA at specific sequences for genetic engineering
3. Household Products:
- Proteases in laundry detergents break down protein stains
- Lipases remove greasy stains
- Amylases help remove starch-based stains
Enzyme Deficiency Disorders
Understanding enzyme deficiencies helps illustrate their importance:
- Lactase deficiency – Cannot digest milk sugar (lactose intolerance)
- Phenylketonuria (PKU) – Cannot break down the amino acid phenylalanine
- Galactosemia – Cannot process galactose from milk
Common Exam Questions and How to Answer Them
Question Type 1: Explaining Enzyme Action
Sample Question: “Explain how enzymes speed up chemical reactions in living organisms.”
Model Answer Structure:
- Define enzymes as biological catalysts
- Explain the active site concept
- Describe enzyme-substrate complex formation
- Mention lowering of activation energy
- Emphasize reusability of enzymes
Question Type 2: Factors Affecting Enzyme Activity
Sample Question: “Describe and explain the effect of temperature on enzyme activity.”
Model Answer Points:
- Low temperature = low kinetic energy = slow reaction
- Optimum temperature = maximum activity
- High temperature = denaturation = loss of function
- Include a graph description
- Mention specific examples (human enzymes at 37°C)
Question Type 3: Comparing Models
Sample Question: “Compare the lock and key model with the induced fit model of enzyme action.”
Key Comparison Points:
- Lock and key: rigid structures, exact fit
- Induced fit: flexible structures, shape changes
- Current scientific acceptance
- Evidence supporting each model
Quick Revision Notes
Key Definitions Box:
- Enzyme: Biological catalyst made of protein
- Active site: Region where substrate binds
- Substrate: Substance acted upon by enzyme
- Product: Result of enzyme-catalyzed reaction
- Denaturation: Permanent loss of enzyme structure and function
- Inhibitor: Substance that decreases enzyme activity
- Optimum conditions: Temperature and pH for maximum enzyme activity
Important Equations Box:
- General enzyme reaction: E + S ⇌ ES → E + P
- Catalase: 2H₂O₂ → 2H₂O + O₂
- Amylase: Starch + Water → Maltose
- Pepsin: Protein + Water → Polypeptides
- Lipase: Fat + Water → Fatty acids + Glycerol
Memory Aids:
- PEST – pH, Enzyme concentration, Substrate concentration, Temperature (factors affecting enzyme activity)
- “Lock and Key is old, Induced Fit is gold” – Remember which model is current
- “Denatured means damaged permanently” – High temperature effects
Common Mistakes to Avoid
1. Conceptual Errors:
- Wrong: “Enzymes are used up in reactions”
- Right: “Enzymes are reusable catalysts”
- Wrong: “All enzymes work best at pH 7”
- Right: “Each enzyme has its own optimal pH”
2. Graph Interpretation Mistakes:
- Not labeling axes properly
- Confusing enzyme concentration with substrate concentration effects
- Forgetting to explain why curves plateau
3. Explanation Errors:
- Using vague terms like “speeds up” without explaining how
- Forgetting to mention the active site in mechanism explanations
- Confusing competitive and non-competitive inhibition
Test Yourself Section
Quick Check Questions:
- What is the name of the region where substrates bind to enzymes?
- Which model of enzyme action is currently accepted by scientists?
- What happens to enzymes at very high temperatures?
- Name three factors that affect enzyme activity.
- What is the difference between competitive and non-competitive inhibition?
Answers:
- Active site
- Induced fit model
- They become denatured (lose their shape and function)
- Temperature, pH, enzyme concentration, substrate concentration, presence of inhibitors
- Competitive inhibition occurs at the active site and can be overcome by increasing substrate concentration; non-competitive inhibition occurs at a different site and cannot be overcome by increasing substrate concentration.
Conclusion: Mastering Enzymes for IGCSE Success
Enzymes are truly the unsung heroes of biology – working tirelessly to make life possible. From the moment you wake up and brush your teeth (with enzyme-containing toothpaste!) to the complex metabolic processes keeping you alive, enzymes are everywhere.
Understanding enzymes isn’t just about passing your IGCSE Biology exam (though this guide will definitely help with that!). It’s about appreciating the incredible molecular machinery that makes life possible and understanding how we can harness these biological catalysts to solve real-world problems.
Related Topics to Explore:
- Topic 6: Plant Nutrition – Enzymes in photosynthesis
- Topic 7: Human Nutrition – Digestive enzymes in detail
- Topic 8: Transport in Plants – Enzymes in active transport
- Topic 12: Respiration – Enzymes in cellular respiration
Remember, biology is all around us, and enzymes are the molecular tools that make it all work. Embrace the complexity, enjoy the learning process, and don’t be afraid to ask questions. Every expert was once a beginner, and with dedication and the right approach, you’ll master this topic and excel in your IGCSE Biology exam.
Good luck with your studies, and remember – just like enzymes make reactions possible, your hard work will make your success inevitable!
Recommended –


1 thought on “IGCSE (Cambridge) Biology Topic 5: Enzymes | Your Complete Study Guide”