Why Equilibrium is Your Gateway to Chemistry Mastery
Have you ever wondered why some chemical reactions seem to “stop” halfway through, leaving both reactants and products in the same container? Welcome to the fascinating world of chemical equilibrium – one of the most crucial concepts in AP Chemistry Unit 7 that bridges the gap between kinetics and thermodynamics.
Equilibrium isn’t just another chapter to memorize – it’s the key to understanding how chemical systems behave in the real world. From the production of ammonia in the Haber process to the regulation of blood pH in your body, equilibrium governs countless processes that keep our world functioning.
The Problem: Most students struggle with equilibrium because they try to memorize formulas without understanding the underlying principles. This leads to confusion during exams and an inability to apply concepts to new situations.
The Solution: This comprehensive guide will transform your understanding of equilibrium from confusing calculations into logical, predictable patterns that you can master.
Equilibrium at a Glance
Essential Formulas
- Equilibrium Constant (Kc): Kc = [Products]^coefficients / [Reactants]^coefficients
- Pressure Equilibrium Constant: Kp = Kc(RT)^Δn
- Reaction Quotient: Q = same expression as K, but at any time
- Relationship: When Q < K (forward reaction favored), Q > K (reverse favored), Q = K (at equilibrium)
Key Principles
- Dynamic Equilibrium: Forward and reverse reaction rates are equal
- Le Chatelier’s Principle: System responds to stress by shifting to relieve it
- Temperature Effect: Only temperature changes K value
- Concentration/Pressure Effects: Shift position but don’t change K
Common Exam Topics (Weightage: ~7-9% of AP Exam)
- Writing equilibrium expressions
- ICE table calculations
- Le Chatelier’s principle applications
- Kp and Kc conversions
- Equilibrium position predictions
Foundation Concepts: Building Your Equilibrium Understanding
What is Chemical Equilibrium?
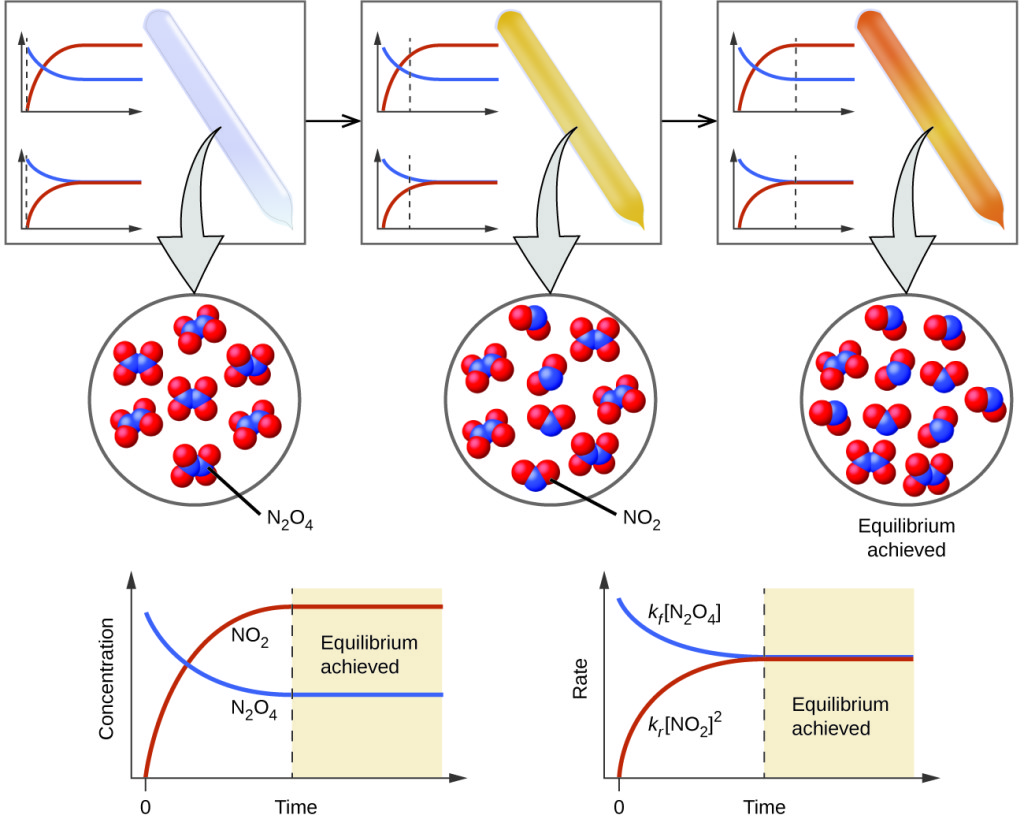
Imagine you’re in a crowded room where people are constantly moving between two areas at exactly the same rate. The number of people in each area stays constant, even though individuals keep moving. This is dynamic equilibrium – the hallmark of chemical systems.
Key Characteristics of Equilibrium:
- Dynamic Nature: Reactions continue in both directions
- Constant Concentrations: Macroscopic properties remain unchanged
- Closed System: No matter exchange with surroundings
- Rate Equality: Rate(forward) = Rate(reverse)
The Equilibrium Constant: Your Chemical GPS
The equilibrium constant (K) is like a GPS for chemical reactions – it tells you exactly where your system wants to go and how far it will travel.
For the general reaction: aA + bB ⇌ cC + dD
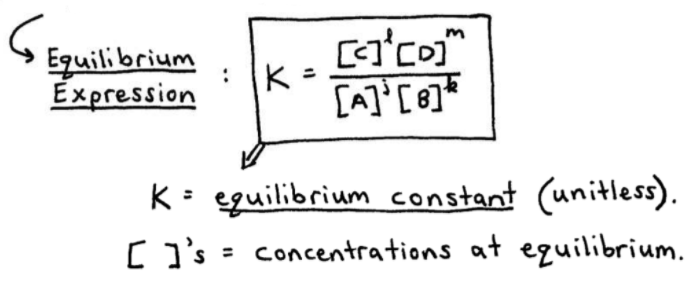
Kc Expression: Kc = [C]^c[D]^d / [A]^a[B]^b
What K Values Tell You:
- K >> 1: Products heavily favored (reaction goes nearly to completion)
- K << 1: Reactants heavily favored (minimal product formation)
- K ≈ 1: Significant amounts of both reactants and products at equilibrium
ICE Tables: Your Problem-Solving Toolkit
ICE tables (Initial, Change, Equilibrium) are your systematic approach to solving equilibrium problems. Think of them as your organized workspace where every piece of information has its place.
ICE Table Structure:
A + B ⇌ C + D
I [Initial concentrations]
C [Changes in concentration]
E [Equilibrium concentrations]Mastering Each Component
1. Writing Equilibrium Expressions
Rules for Writing K Expressions:
- Include only aqueous and gaseous species
- Exclude pure solids and liquids
- Use concentrations for Kc, partial pressures for Kp
- Raise each term to the power of its stoichiometric coefficient
Example: For the reaction 2SO₂(g) + O₂(g) ⇌ 2SO₃(g)
Kc = [SO₃]² / ([SO₂]²[O₂])
Common Mistakes to Avoid:
Including solids/liquids in the expression
Using molarity instead of partial pressure for Kp
Forgetting to raise terms to their coefficients
2. Le Chatelier’s Principle: Predicting System Response
Le Chatelier’s principle is like Newton’s third law for chemistry – every action has an equal and opposite reaction.
Stress Factors and System Response:
Concentration Changes:
- Adding reactant → Shift right (toward products)
- Adding product → Shift left (toward reactants)
- Removing reactant → Shift left
- Removing product → Shift right
Temperature Changes:
- Exothermic reactions: Higher temperature shifts left (K decreases)
- Endothermic reactions: Higher temperature shifts right (K increases)
Pressure Changes (for gaseous systems):
- Increased pressure → Shift toward fewer moles of gas
- Decreased pressure → Shift toward more moles of gas
3. Relationship Between Kp and Kc
For reactions involving gases, you’ll work with both concentration (Kc) and pressure (Kp) equilibrium constants.
Key Relationship: Kp = Kc(RT)^Δn
Where:
- R = 0.08206 L·atm/(mol·K)
- T = temperature in Kelvin
- Δn = (moles of gaseous products) – (moles of gaseous reactants)
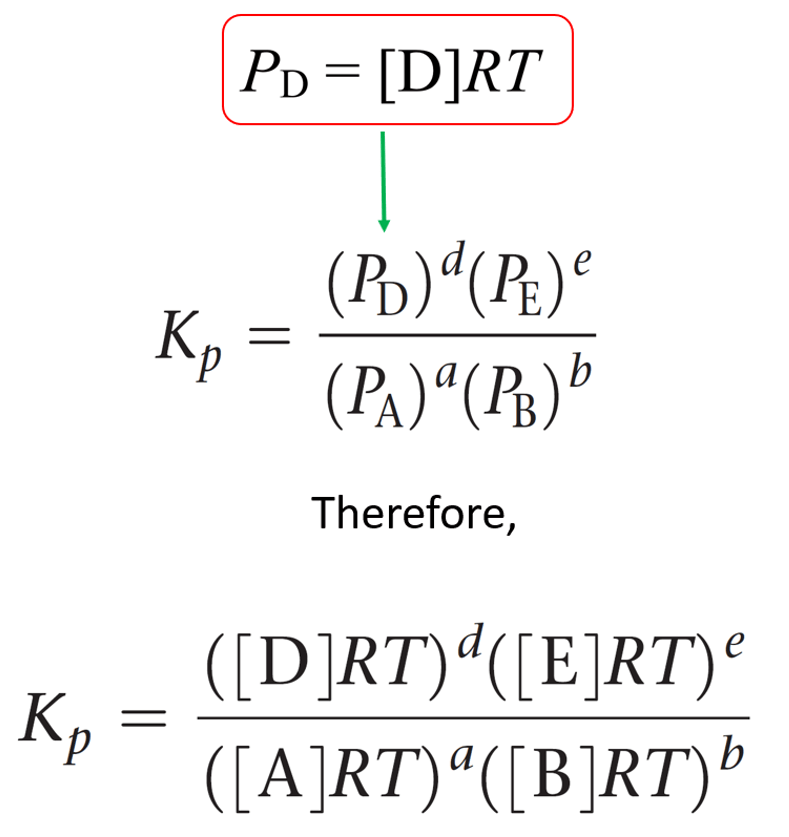
Practice Makes Perfect
20 Multiple Choice Practice Questions
Questions 1-5: Equilibrium Expression Writing
1. For the reaction CaCO₃(s) ⇌ CaO(s) + CO₂(g), the equilibrium expression is:
A) Kc = [CaO][CO₂]/[CaCO₃]
B) Kc = [CO₂]
C) Kc = [CaCO₃]/[CaO][CO₂]
D) Kc = 1/[CO₂]
Answer: B – Solids are not included in equilibrium expressions.
2. For 2NO₂(g) ⇌ N₂O₄(g), if Kc = 4.0 at 25°C, what is Kc for N₂O₄(g) ⇌ 2NO₂(g)?
A) 4.0
B) 2.0
C) 0.25
D) 16
Answer: C – When reversing a reaction, K becomes 1/K.
3. The equilibrium constant for A + B ⇌ C is K₁. For 2A + 2B ⇌ 2C, the equilibrium constant is:
A) K₁
B) 2K₁
C) K₁²
D) K₁/2
Answer: C – When multiplying coefficients by n, K becomes K^n.
4. Which of the following is NOT included in an equilibrium expression?
A) Dissolved ions
B) Gaseous molecules
C) Pure liquids
D) Aqueous compounds
Answer: C – Pure solids and liquids are excluded.
5. For the reaction H₂(g) + I₂(g) ⇌ 2HI(g), the equilibrium expression is:
A) Kc = [HI]/[H₂][I₂]
B) Kc = [HI]²/[H₂][I₂]
C) Kc = [H₂][I₂]/[HI]²
D) Kc = 2[HI]/[H₂][I₂]
Answer: B – Products in numerator, reactants in denominator, raised to coefficients.
Questions 6-10: Le Chatelier’s Principle
6. For the exothermic reaction N₂ + 3H₂ ⇌ 2NH₃ + heat, increasing temperature will:
A) Increase K and shift right
B) Decrease K and shift left
C) Increase K and shift left
D) Decrease K and shift right
Answer: B – Higher temperature favors endothermic direction (left).
7. In the same reaction, adding more N₂ will:
A) Increase K value
B) Decrease K value
C) Shift equilibrium right without changing K
D) No effect on equilibrium
Answer: C – Adding reactant shifts right, but K is unchanged.
8. Increasing pressure in 2SO₂(g) + O₂(g) ⇌ 2SO₃(g) will:
A) Shift left toward reactants
B) Shift right toward products
C) No effect
D) Increase K value
Answer: B – Higher pressure favors fewer moles of gas (3 moles → 2 moles).
9. For PCl₅(g) ⇌ PCl₃(g) + Cl₂(g), decreasing volume will:
A) Shift right
B) Shift left
C) No effect
D) Change K value
Answer: B – Decreased volume = increased pressure, favoring fewer moles (left side).
10. A catalyst added to an equilibrium system will:
A) Shift equilibrium right
B) Shift equilibrium left
C) Increase K value
D) No effect on equilibrium position
Answer: D – Catalysts speed up both directions equally, no net shift.
Questions 11-15: Calculations and ICE Tables
11. For the reaction A ⇌ B, if [A]₀ = 1.0 M and at equilibrium [A] = 0.6 M, then [B]eq is:
A) 0.6 M
B) 0.4 M
C) 1.0 M
D) 1.6 M
Answer: B – Conservation of mass: what A loses, B gains.
12. If Q > K for a reaction, the system will:
A) Be at equilibrium
B) Shift right
C) Shift left
D) Remain unchanged
Answer: C – Q > K means too much product, shift left to reactants.
13. For H₂ + I₂ ⇌ 2HI, if Kc = 50 and [H₂] = [I₂] = 0.1 M at equilibrium, [HI] equals:
A) 0.22 M
B) 0.71 M
C) 5.0 M
D) 0.50 M
Answer: B – Kc = [HI]²/[H₂][I₂] = [HI]²/(0.1)(0.1) = 50, so [HI] = √(50 × 0.01) = 0.71 M
14. At 25°C, Kc = 4.0 for 2NO₂ ⇌ N₂O₄. What is Kp if R = 0.0821?
A) 4.0
B) 0.16
C) 98
D) 0.041
Answer: B – Δn = 1-2 = -1, Kp = Kc(RT)^Δn = 4.0(0.0821×298)^(-1) = 0.16
15. If Kc = 1.0 × 10⁻⁴ for a reaction, this indicates:
A) Products are highly favored
B) Reactants are highly favored
C) Equal amounts of reactants and products
D) The reaction is very fast
Answer: B – Small K value means reactants are favored.
Questions 16-20: Advanced Applications
16. For CaF₂(s) ⇌ Ca²⁺(aq) + 2F⁻(aq), the equilibrium expression is:
A) Ksp = [Ca²⁺][F⁻]²
B) Ksp = [Ca²⁺][F⁻]²/[CaF₂]
C) Ksp = [Ca²⁺][2F⁻]
D) Ksp = [Ca²⁺] + [F⁻]²
Answer: A – Solubility product expression excludes solid.
17. Which factor will change the value of K?
A) Adding a catalyst
B) Changing pressure
C) Changing temperature
D) Changing concentration
Answer: C – Only temperature changes K value.
18. For the gas-phase reaction 2A ⇌ B + C, if the total pressure increases by a factor of 2, the equilibrium will:
A) Shift right
B) Shift left
C) Not shift
D) Shift right then left
Answer: B – Higher pressure favors fewer moles (2 moles vs 2 moles… wait, equal moles means no shift – Answer: C)
19. If Kc = 25 for A + B ⇌ C + D, and initial [A] = [B] = 1.0 M, [C] = [D] = 0, the equilibrium [A] is approximately:
A) 0.17 M
B) 0.83 M
C) 0.50 M
D) 0.33 M
Answer: A – Using ICE table and quadratic formula.
20. The principle that explains why equilibrium shifts when stressed is:
A) Hess’s law
B) Le Chatelier’s principle
C) Dalton’s law
D) Raoult’s law
Answer: B – Le Chatelier’s principle governs equilibrium shifts.
5 Free Response Problems with Step-by-Step Solutions
Problem 1: ICE Table Calculation
Question: At 1000 K, Kc = 2.0 × 10⁻⁴ for the reaction CO₂(g) + H₂(g) ⇌ CO(g) + H₂O(g). If 1.0 mol CO₂ and 1.0 mol H₂ are placed in a 5.0 L container, what are the equilibrium concentrations?
Solution:
Step 1: Write the equilibrium expression
Kc = [CO][H₂O]/[CO₂][H₂] = 2.0 × 10⁻⁴
Step 2: Calculate initial concentrations
[CO₂]₀ = [H₂]₀ = 1.0 mol/5.0 L = 0.20 M
[CO]₀ = [H₂O]₀ = 0 M
Step 3: Set up ICE table
CO₂ H₂ CO H₂O
I 0.20 0.20 0 0
C -x -x +x +x
E 0.20-x 0.20-x x xStep 4: Substitute into Kc expression
2.0 × 10⁻⁴ = (x)(x)/[(0.20-x)(0.20-x)]
2.0 × 10⁻⁴ = x²/(0.20-x)²
Step 5: Solve (using quadratic approximation since K is small)
Assume x << 0.20, so (0.20-x) ≈ 0.20
2.0 × 10⁻⁴ = x²/(0.20)²
x² = 2.0 × 10⁻⁴ × 0.04 = 8.0 × 10⁻⁶
x = 2.8 × 10⁻³ M
Step 6: Check approximation and calculate final concentrations
2.8 × 10⁻³ << 0.20 ✓ (approximation valid)
Final Answer:
[CO₂] = [H₂] = 0.20 – 0.0028 = 0.197 M ≈ 0.20 M
[CO] = [H₂O] = 2.8 × 10⁻³ M
Problem 2: Le Chatelier’s Principle Application
Question: Consider the exothermic reaction: 2SO₂(g) + O₂(g) ⇌ 2SO₃(g) + heat. Explain what happens to the equilibrium position and K value when: (a) Temperature is increased, (b) SO₂ is added, (c) A catalyst is added, (d) The volume is decreased.
Solution:
(a) Temperature increase:
- Equilibrium shift: Left (toward reactants)
- Reasoning: Higher temperature favors endothermic direction
- K value: Decreases (only temperature changes K)
(b) SO₂ added:
- Equilibrium shift: Right (toward products)
- Reasoning: Le Chatelier’s principle – system relieves stress by consuming added SO₂
- K value: Unchanged (concentration changes don’t affect K)
(c) Catalyst added:
- Equilibrium shift: No shift
- Reasoning: Catalyst speeds both forward and reverse reactions equally
- K value: Unchanged
(d) Volume decreased (pressure increased):
- Equilibrium shift: Right (toward products)
- Reasoning: Higher pressure favors side with fewer gas molecules (3 moles → 2 moles)
- K value: Unchanged (Kc unaffected by pressure changes)
Problem 3: Kp and Kc Conversion
Question: For the reaction N₂O₄(g) ⇌ 2NO₂(g), Kc = 4.6 × 10⁻³ at 25°C. Calculate Kp.
Solution:
Step 1: Identify the relationship
Kp = Kc(RT)^Δn
Step 2: Calculate Δn
Δn = (moles gaseous products) – (moles gaseous reactants)
Δn = 2 – 1 = 1
Step 3: Convert temperature to Kelvin
T = 25°C + 273 = 298 K
Step 4: Use R = 0.08206 L·atm/(mol·K)
Step 5: Calculate Kp
Kp = (4.6 × 10⁻³)(0.08206 × 298)¹
Kp = (4.6 × 10⁻³)(24.45)
Kp = 0.11
Final Answer: Kp = 0.11 atm
Problem 4: Equilibrium Position Prediction
Question: For the reaction H₂(g) + I₂(g) ⇌ 2HI(g), Kc = 50 at 445°C. If a mixture contains [H₂] = 0.10 M, [I₂] = 0.20 M, and [HI] = 1.0 M, determine if the system is at equilibrium. If not, predict the direction of shift.
Solution:
Step 1: Calculate reaction quotient Q
Q = [HI]²/[H₂][I₂] = (1.0)²/(0.10)(0.20) = 1.0/0.020 = 50
Step 2: Compare Q with K
Q = 50 and K = 50
Therefore, Q = K
Step 3: Determine equilibrium status
Since Q = K, the system is at equilibrium.
Final Answer: The system is at equilibrium; no net reaction occurs.
Problem 5: Complex ICE Table with Quadratic
Question: For the reaction 2HI(g) ⇌ H₂(g) + I₂(g), Kc = 0.020 at 445°C. If 2.0 mol HI is placed in a 1.0 L container, find the equilibrium concentrations.
Solution:
Step 1: Write equilibrium expression
Kc = [H₂][I₂]/[HI]² = 0.020
Step 2: Calculate initial concentration
[HI]₀ = 2.0 mol/1.0 L = 2.0 M
Step 3: Set up ICE table
2HI H₂ I₂
I 2.0 0 0
C -2x +x +x
E 2.0-2x x xStep 4: Substitute into Kc expression
0.020 = (x)(x)/(2.0-2x)²
Step 5: Solve quadratic equation
0.020 = x²/(2.0-2x)²
√0.020 = x/(2.0-2x)
0.141 = x/(2.0-2x)
0.141(2.0-2x) = x
0.282 – 0.282x = x
0.282 = 1.282x
x = 0.22 M
Step 6: Calculate equilibrium concentrations
[H₂] = [I₂] = 0.22 M
[HI] = 2.0 – 2(0.22) = 1.56 M
Step 7: Verify answer
Kc = (0.22)(0.22)/(1.56)² = 0.0484/2.43 = 0.020 ✓
Final Answer:
[HI] = 1.56 M, [H₂] = [I₂] = 0.22 M
Study Strategies and Exam Preparation Tips
Memory Techniques for Success
1. The RICE Method for ICE Tables
- Reaction setup
- Initial concentrations
- Change in concentrations
- Equilibrium concentrations
2. Le Chatelier’s Mnemonic: “STOP”
- Stress the system
- Take action to relieve stress
- Opposite direction shift
- Predict new equilibrium
3. K Value Memory Device
- K > 1: “Products Prefer to Party” (products favored)
- K < 1: “Reactants Refuse to React” (reactants favored)
- K ≈ 1: “Balanced Battle” (equal concentrations)
Time Management for AP Exam
Multiple Choice Section (45 minutes, 60 questions)
- Equilibrium questions: Expect 4-6 questions (7-10% of exam)
- Time per question: 45 seconds average
- Strategy: Skip complex calculations initially, return later
Free Response Section (105 minutes, 7 questions)
- Long questions (10 points each): 15 minutes per question
- Short questions (4 points each): 6 minutes per question
- Equilibrium typically appears: 1 long question or 2 short questions per exam
Common Error Analysis
Top 5 Student Mistakes:
1. Including solids/liquids in K expressions
Wrong: Kc = [CO₂]/[CaCO₃] for CaCO₃(s) ⇌ CaO(s) + CO₂(g)
Correct: Kc = [CO₂]
2. Confusing Q and K relationships
Wrong: “Q < K means shift left”
Correct: “Q < K means shift right (toward products)”
3. Temperature effect on K
Wrong: “Higher temperature always increases K”
Correct: “Higher temperature increases K for endothermic, decreases K for exothermic”
4. Pressure effects on equilibria
Wrong: “Pressure always shifts toward products”
Correct: “Pressure shifts toward fewer moles of gas”
5. ICE table sign conventions
Wrong: Using positive changes for reactants
Correct: Negative changes for consumed species, positive for produced
Study Schedule (4-Week Plan)
Week 1: Foundation Building
- Day 1-2: Equilibrium concept and dynamic nature
- Day 3-4: Writing K expressions
- Day 5-6: Simple ICE table calculations
- Day 7: Practice test
Week 2: Advanced Calculations
- Day 1-2: Complex ICE tables and quadratics
- Day 3-4: Kp and Kc conversions
- Day 5-6: Le Chatelier’s principle
- Day 7: Mixed problem practice
Week 3: Application and Analysis
- Day 1-2: Solubility equilibria
- Day 3-4: Acid-base equilibria preview
- Day 5-6: Real-world applications
- Day 7: Timed practice exam
Week 4: Review and Refinement
- Day 1-2: Error analysis and correction
- Day 3-4: Speed practice
- Day 5-6: Final review
- Day 7: Rest and confidence building
Advanced Applications and Real-World Connections
Industrial Applications
1. Haber Process (Ammonia Production)
N₂(g) + 3H₂(g) ⇌ 2NH₃(g) + heat
Industrial Optimization:
- High pressure: Favors fewer moles (4 → 2)
- Moderate temperature: Balance between rate and equilibrium position
- Catalyst: Iron-based to increase reaction rate
- Continuous removal: Le Chatelier shifts right
Economic Impact: $80 billion global ammonia market for fertilizers
2. Contact Process (Sulfuric Acid Production)
2SO₂(g) + O₂(g) ⇌ 2SO₃(g)
Process Conditions:
- Temperature: 450°C (compromise between rate and equilibrium)
- Pressure: 2-3 atm (minimal effect due to small Δn)
- Catalyst: Vanadium pentoxide (V₂O₅)
Biological Systems
1. Oxygen Transport (Hemoglobin Equilibrium)
Hb(aq) + 4O₂(g) ⇌ Hb(O₂)₄(aq)
Physiological Factors:
- Lung conditions: High pO₂ shifts right (oxygen binding)
- Tissue conditions: Low pO₂ shifts left (oxygen release)
- Carbon monoxide poisoning: CO competes with O₂, deadly equilibrium shift
2. Blood pH Regulation (Bicarbonate Buffer)
CO₂(g) + H₂O(l) ⇌ H₂CO₃(aq) ⇌ H⁺(aq) + HCO₃⁻(aq)
pH Control Mechanisms:
- Respiratory: Change CO₂ levels through breathing rate
- Renal: Adjust HCO₃⁻ excretion/retention
- Emergency response: Hyperventilation removes CO₂, shifts left, increases pH
Environmental Applications
1. Ocean Acidification
CO₂(g) + H₂O(l) ⇌ H₂CO₃(aq) ⇌ H⁺(aq) + HCO₃⁻(aq)
Climate Change Impact:
- Increased atmospheric CO₂ → more dissolved CO₂
- Le Chatelier shifts right → lower ocean pH
- Consequences: Coral bleaching, shell dissolution
2. Atmospheric Chemistry
2NO₂(g) ⇌ N₂O₄(g) + heat
Smog Formation:
- Hot days: Higher temperature shifts left, more brown NO₂
- Cool nights: Lower temperature shifts right, colorless N₂O₄
- Visibility impact: NO₂ concentration affects air quality
Pharmaceutical Applications
Drug Solubility and Bioavailability
Drug(s) ⇌ Drug(aq)
Formulation Strategies:
- pH adjustment: Optimize ionization equilibria
- Salt formation: Alter solubility equilibrium
- Complexation: Shift equilibrium toward dissolved form
FAQs: Conceptual Understanding
Q1: Why does adding a catalyst not shift equilibrium position?
A: A catalyst provides an alternative reaction pathway with lower activation energy for both forward and reverse reactions. Since it speeds up both directions equally, the ratio of forward to reverse rates remains unchanged at equilibrium. Think of it as upgrading both lanes of a two-way highway equally – traffic flows faster in both directions, but the net flow remains zero.
Q2: How can I remember which way pressure shifts affect equilibrium?
A: Use the “mole counting method”:
- Count moles of gas on each side
- Higher pressure = system wants fewer moles
- Equilibrium shifts toward the side with fewer gas molecules
- If moles are equal, pressure has no effect
Memory device: “Pressure Prefers Petite” (smaller number of moles)
Q3: Why does only temperature change the K value?
A: K is fundamentally related to the energy difference between reactants and products. Temperature affects the distribution of molecular energies (Maxwell-Boltzmann distribution), changing the fraction of molecules with sufficient energy to react. Other factors (concentration, pressure) only affect the rate at which equilibrium is reached, not the energy relationships that determine the equilibrium position.
Problem-Solving Strategies
Q4: When should I use the quadratic formula versus approximations in ICE table problems?
A: Use this decision tree:
- Calculate x/initial concentration ratio from the approximation
- If x/[initial] < 5%: Approximation is valid
- If x/[initial] > 5%: Use quadratic formula
- Always verify your final answer by substituting back into K expression
Q5: How do I approach multi-step equilibrium problems?
A: Follow the systematic approach:
- Identify all equilibria in the system
- Write all K expressions
- Determine which equilibrium controls the system
- Solve step-by-step, using results from one equilibrium in the next
- Check for consistency across all equilibria
Q6: What’s the difference between reaction quotient Q and equilibrium constant K?
A:
- K: Calculated using equilibrium concentrations, constant at given temperature
- Q: Calculated using any concentrations, tells you direction of reaction
- Comparison:
- Q < K: Reaction proceeds forward (toward products)
- Q > K: Reaction proceeds reverse (toward reactants)
- Q = K: System at equilibrium
Exam Strategy
Q7: How should I manage time on equilibrium problems during the AP exam?
A:
Multiple Choice:
- Skip complex calculations initially
- Look for conceptual shortcuts
- Use elimination strategies
- Return to calculations if time permits
Free Response:
- Read entire question first
- Identify what’s being asked
- Show all work clearly
- Use proper significant figures
- Check units in final answers
Q8: What are the most commonly tested equilibrium concepts?
A: Based on recent AP exams:
- Le Chatelier’s principle applications (30% of equilibrium questions)
- ICE table calculations (25%)
- Writing K expressions (20%)
- Kp/Kc conversions (15%)
- Q vs K predictions (10%)
Q9: How can I avoid sign errors in ICE tables?
A: Use this systematic approach:
- Always use the balanced equation as your guide
- Reactants decrease: Always negative changes (-x, -2x, etc.)
- Products increase: Always positive changes (+x, +2x, etc.)
- Double-check stoichiometry: If 2 moles of A react, write -2x, not -x
- Verify mass conservation: Total atoms in = Total atoms out
Common Misconceptions
Q10: Do solids and liquids never affect equilibrium?
A: Clarification: Pure solids and liquids are not included in K expressions because their concentrations are constant. However:
- They DO participate in the reaction
- Surface area can affect reaction rate (though not equilibrium position)
- Amount present must be sufficient for the reaction
- Phase changes can shift equilibrium if pure phases appear/disappear
Conclusion: Your Path to Equilibrium Mastery
Congratulations! You’ve now explored the complete landscape of chemical equilibrium in AP Chemistry Unit 7. From the fundamental concept of dynamic balance to complex industrial applications, you’ve built a comprehensive understanding that will serve you well beyond the AP exam.
Key Takeaways for Success
Conceptual Mastery:
- Equilibrium is dynamic: Never forget that molecular-level activity continues even when macroscopic properties remain constant
- Le Chatelier’s principle: Your universal tool for predicting system responses to stress
- K values tell stories: Large K (products win), small K (reactants win), K≈1 (close battle)
Problem-Solving Excellence:
- ICE tables are your friend: Systematic organization prevents errors
- Approximations save time: Use when K is small and initial concentrations are large
- Check your work: Always substitute final answers back into original expressions
Exam Strategy Success:
- Pattern recognition: Identify question types quickly
- Time management: Balance speed with accuracy
- Show your work: Partial credit can save your score
Next Steps in Your Chemistry Journey
Immediate Actions (Next 1-2 Weeks):
- Complete additional practice: Seek out past AP exam questions
- Form study groups: Explain equilibrium concepts to classmates
- Create summary sheets: Distill key formulas and concepts
- Time yourself: Practice under exam conditions
Unit Connections to Explore:
- Unit 8 (Acids and Bases): Equilibrium principles applied to proton transfer
- Unit 9 (Applications of Thermodynamics): Energy relationships that determine K values
- Real-world research: Investigate industrial processes using equilibrium principles
Advanced Learning Opportunities:
- Research projects: Ocean acidification, atmospheric chemistry, pharmaceutical development
- Laboratory extensions: Design experiments to test Le Chatelier’s principle
- Career connections: Chemical engineering, environmental science, biochemistry
Final Motivation
Remember that mastering equilibrium is not just about memorizing formulas or solving problems-it’s about developing a deep appreciation for the dynamic balance that governs chemical systems throughout our universe. From the oxygen-carrying capacity of your blood to the industrial production of fertilizers that feed billions, equilibrium principles are working around you every moment.
Every time you breathe, your body demonstrates Le Chatelier’s principle as hemoglobin responds to changing oxygen concentrations. Every can of soda you open releases CO₂ according to Henry’s law equilibrium. Every antacid you take neutralizes stomach acid through acid-base equilibrium.
You’re not just studying chemistry-you’re learning to understand the molecular choreography of life itself.
Your Success Formula
Confidence + Practice + Understanding = AP Chemistry Success
As you continue your preparation, remember that equilibrium mastery comes through consistent practice and deep thinking, not last-minute cramming. Trust in the systematic approaches you’ve learned, apply Le Chatelier’s wisdom to your own learning process, and maintain the dynamic balance between focused study and adequate rest.
Your equilibrium constant for success is as high as you make it. The reaction is:
Preparation + Effort ⇌ Mastery + Confidence
And with the right conditions (consistent study, positive mindset, strategic practice), this equilibrium lies far to the right.
Good luck with your AP Chemistry exam-you’ve got the tools, knowledge, and strategies to succeed!
Additional Resources for Continued Learning
Recommended Practice Materials
- College Board AP Central: Official practice exams and scoring guidelines
- Khan Academy: Free video explanations and practice problems
- AP Chemistry Prep Books: Princeton Review, Barron’s, 5 Steps to a 5
- Online Simulations: PhET Interactive Simulations for equilibrium concepts
Professional Development
- American Chemical Society (ACS): Student membership and resources
- Research Opportunities: Summer programs at local universities
- Chemistry Competitions: USNCO (US National Chemistry Olympiad)
- Career Exploration: ChemClub activities and chemistry career resources
Your journey in chemistry is just beginning, and equilibrium principles will serve as a foundation for everything that follows. Keep questioning, keep practicing, and keep discovering the elegant simplicity underlying the complex chemical world around us.
The equilibrium between learning and mastery lies ahead-shift it in your favor!
Read More –

1 thought on “Master AP Chemistry Unit 7: Equilibrium – Your Complete Guide to Acing Chemical Equilibrium”