Why Experimental Chemistry Matters More Than You Think
Imagine you’re a detective solving a mystery, but instead of fingerprints and witnesses, you’re using test tubes and chemical reactions. That’s exactly what Experimental techniques and chemical analysis is all about! Every time you identify an unknown substance or measure the exact amount of acid needed to neutralize a base, you’re becoming a chemical detective.
Topic 12 of IGCSE Chemistry isn’t just about memorizing procedures – it’s about understanding how chemists actually work in the real world. From testing water purity in your local treatment plant to developing new medicines in pharmaceutical labs, these experimental techniques are the backbone of modern chemistry.
Whether you love hands-on practical work or find it challenging, this comprehensive guide will transform you into a confident chemistry experimentalist. We’ll break down every concept, share insider tips, and help you tackle those tricky exam questions with ease.
Understanding the Foundation: What is Chemical Analysis?
Chemical analysis is essentially asking two fundamental questions about any substance:
- What is it? (Qualitative Analysis)
- How much is there? (Quantitative Analysis)
Think of it like examining a mystery smoothie. Qualitative analysis tells you it contains strawberries, bananas, and yogurt, while quantitative analysis tells you there are 50g of strawberries, 30g of bananas, and 100ml of yogurt.
The Two Pillars of Chemical Analysis
Qualitative Analysis: The “What” Detective Work
- Identifies the presence or absence of specific substances
- Uses characteristic reactions, colors, and precipitation
- Like a chemical fingerprint system
Quantitative Analysis: The “How Much” Measurement
- Determines exact amounts or concentrations
- Uses precise measurements and calculations
- Requires mathematical skills alongside chemistry knowledge
1: Mastering Qualitative Analysis
Metal Ion Identification: Your Chemical Rainbow
One of the most colorful aspects of chemistry is identifying metal ions through their characteristic reactions. It’s like having a secret code where each metal has its unique signature.
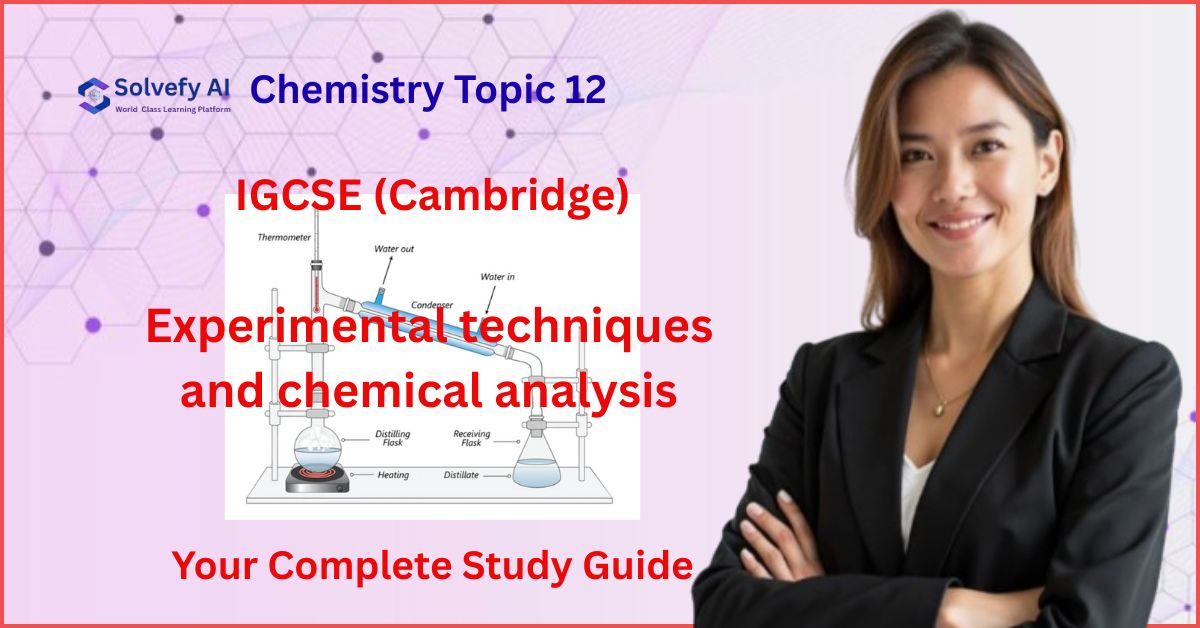
Flame Tests: The Fireworks Method
When metal ions are heated in a flame, they produce distinctive colors:
Essential Flame Test Colors:
- Lithium (Li⁺): Crimson red – Think “Lithium = Lipstick red”
- Sodium (Na⁺): Golden yellow – Remember “Sodium streetlights”
- Potassium (K⁺): Lilac/violet – “King’s purple”
- Calcium (Ca²⁺): Orange-red – “Calcium = Carrot orange”
- Copper (Cu²⁺): Blue-green – “Copper = Blue-green patina”
Memory Tip: Create the acronym “Little Naughty Kids Can Cook” for Li, Na, K, Ca, Cu!
Step-by-Step Flame Test Procedure:
- Clean the nichrome wire by dipping in dilute HCl and heating until no color appears
- Dip the clean wire into the sample
- Hold the wire in the hottest part of the flame
- Observe and record the color immediately
- Clean the wire before testing the next sample
Hydroxide Precipitation Tests
When you add sodium hydroxide (NaOH) to metal ion solutions, magic happens – different colored precipitates form!

Key Hydroxide Precipitate Reactions:
Iron(II) – Fe²⁺:
Fe²⁺ + 2OH⁻ → Fe(OH)₂ (green precipitate)
The precipitate turns brown on standing due to oxidation
Iron(III) – Fe³⁺:
Fe³⁺ + 3OH⁻ → Fe(OH)₃ (red-brown precipitate)
Copper – Cu²⁺:
Cu²⁺ + 2OH⁻ → Cu(OH)₂ (blue precipitate)
Zinc – Zn²⁺:
Zn²⁺ + 2OH⁻ → Zn(OH)₂ (white precipitate)
This precipitate dissolves in excess NaOH
Pro Tip: Remember “Iron(III) = rust-brown, just like real rust!”
Anion Identification: Testing for Negative Ions
Carbonate Test (CO₃²⁻):
The classic “fizz test” – add dilute acid and listen for effervescence!
CO₃²⁻ + 2H⁺ → CO₂ + H₂O
Test the gas produced with limewater – it turns milky white:
CO₂ + Ca(OH)₂ → CaCO₃ + H₂O
Sulfate Test (SO₄²⁻):
Add barium chloride solution – a white precipitate of barium sulfate forms:
Ba²⁺ + SO₄²⁻ → BaSO₄ (white precipitate)
Chloride Test (Cl⁻):
Add silver nitrate solution – a white precipitate of silver chloride forms:
Ag⁺ + Cl⁻ → AgCl (white precipitate)
Memory Device: “Barium Blocks Sulfates, Silver Stops Chlorides”
2: Mastering Quantitative Analysis
Understanding Concentration: The Recipe Principle
Imagine making orange squash – the more concentrated your mixture, the stronger the flavor. Chemical concentration works the same way!
Key Concentration Units:
- g/dm³ (grams per cubic decimeter): Mass of solute in 1000 cm³ of solution
- mol/dm³ (moles per cubic decimeter): Number of moles of solute in 1000 cm³ of solution
Essential Formula:
Concentration (g/dm³) = Mass of solute (g) / Volume of solution (dm³)
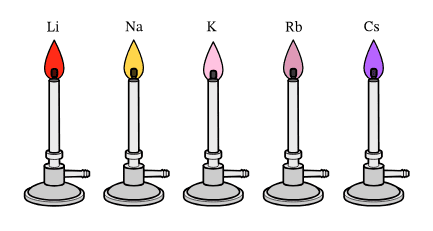
Titration: The Precision Game
Titration is like finding the perfect balance point – it’s where chemistry meets mathematics in beautiful harmony.
The Titration Process Explained:
Equipment You’ll Need:
- Burette (for accurate volume measurement of titrant)
- Pipette (for precise volume of analyte)
- Conical flask (for the reaction)
- Indicator (to show the end point)
Step-by-Step Titration Method:
- Preparation: Fill the burette with your titrant (known concentration)
- Measurement: Use a pipette to transfer exactly 25.0 cm³ of analyte to the conical flask
- Indication: Add 2-3 drops of suitable indicator
- Titration: Add titrant drop by drop until the end point is reached
- Recording: Note the volume of titrant used
Choosing the Right Indicator:
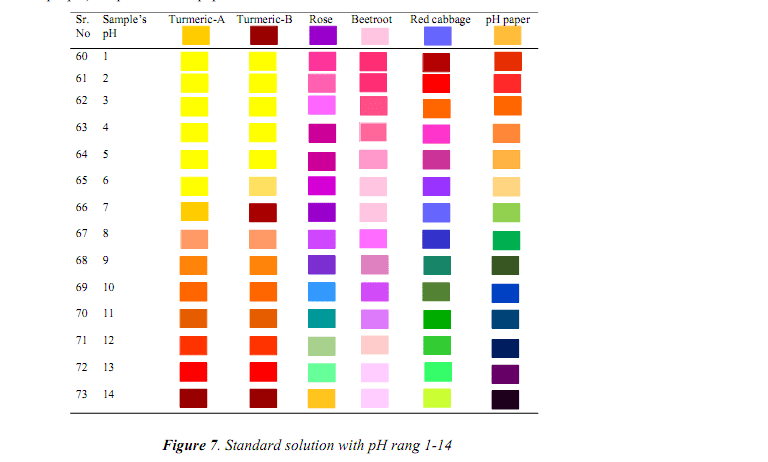
- Methyl orange: Red in acid, yellow in alkali (pH 3.1-4.4)
- Phenolphthalein: Colorless in acid, pink in alkali (pH 8.3-10.0)
- Universal indicator: Shows pH through color spectrum
Titration Calculations Made Simple:
The Golden Formula:
n₁V₁ = n₂V₂
Where:
- n = number of moles in balanced equation
- V = volume in dm³
Example Problem:
25.0 cm³ of 0.1 mol/dm³ NaOH neutralizes 20.0 cm³ of HCl. Find the concentration of HCl.
NaOH + HCl → NaCl + H₂O (1:1 ratio)
Using n₁V₁ = n₂V₂:
1 × 0.025 × 0.1 = 1 × 0.020 × C₂
C₂ = 0.125 mol/dm³
Volumetric Analysis: Precision in Practice
Key Volumetric Techniques:
1. Acid-Base Titrations:
- Strong acid vs strong base: Sharp end point, any indicator works
- Weak acid vs strong base: Use phenolphthalein
- Strong acid vs weak base: Use methyl orange
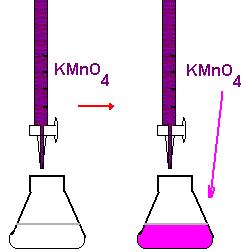
2. Redox Titrations:
Often self-indicating (like potassium manganate(VII) turning from purple to colorless)
3: Essential Laboratory Techniques
Accurate Measurement Techniques
Using a Burette Like a Pro:
- Rinse with distilled water, then with the solution you’ll use
- Fill above the zero mark, then run solution out to exactly zero
- Remove air bubbles from the tap
- Read at eye level to the bottom of the meniscus
- Record to 2 decimal places (e.g., 23.45 cm³)
Pipette Precision:
- Rinse with the solution being measured
- Fill above the graduation mark
- Use a pipette filler (never suck by mouth!)
- Allow to drain vertically with the tip touching the vessel wall
Memory Tip: “Rinse, Fill, Read, Record” – the 4 R’s of accurate measurement!
Separation Techniques
Crystallization: Growing Your Own Crystals
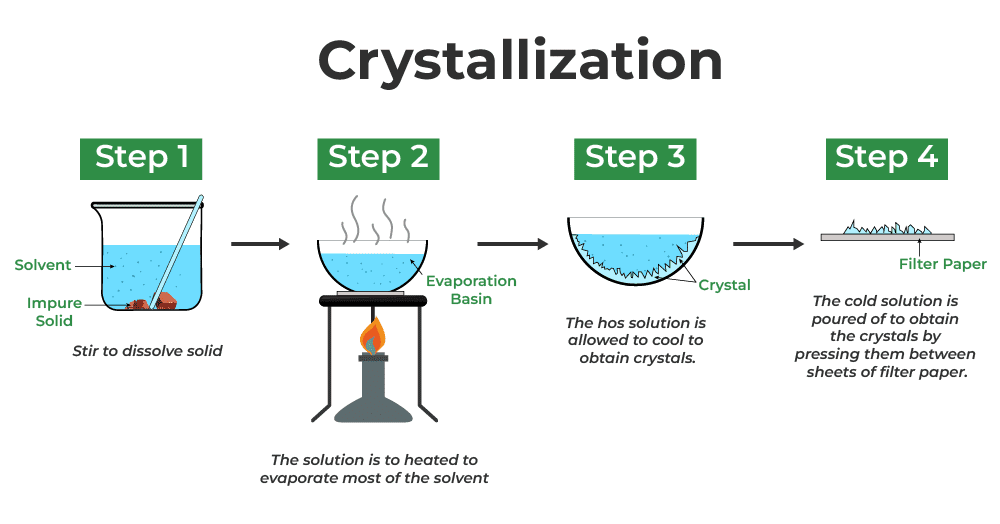
Perfect for separating soluble solids from solutions:
- Heat the solution to concentrate it
- Allow to cool slowly
- Filter to collect crystals
- Wash with cold distilled water
- Dry in a warm oven
Distillation: The Purification Master
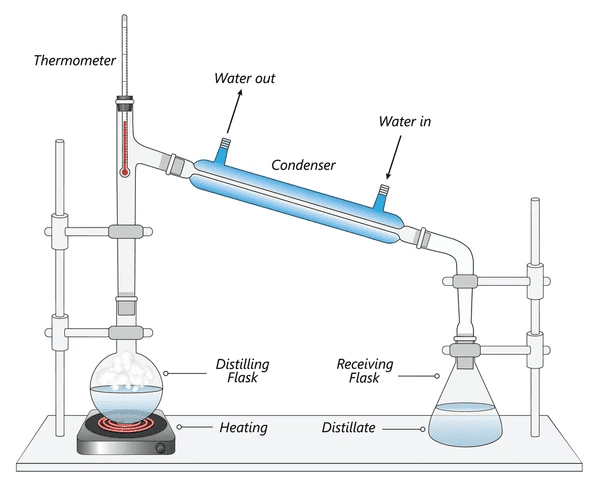
Used for separating liquids with different boiling points:
- Simple distillation: For large boiling point differences (>25°C)
- Fractional distillation: For similar boiling points
4: Gas Collection and Analysis
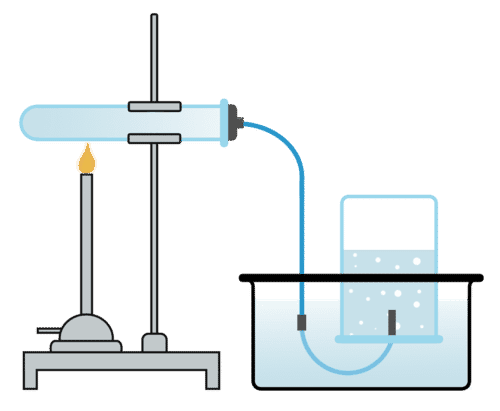
Methods of Gas Collection
1. Displacement of Water:
For gases that don’t dissolve in water (H₂, O₂, CO₂)
2. Downward Delivery:
For gases denser than air (CO₂, Cl₂)
3. Upward Delivery:
For gases less dense than air (NH₃, H₂)
Gas Testing Procedures
Essential Gas Tests:
- Hydrogen: Burns with a ‘pop’ sound with a lighted splint
- Oxygen: Relights a glowing splint
- Carbon dioxide: Turns limewater milky
- Ammonia: Turns red litmus paper blue
- Chlorine: Bleaches damp litmus paper
Key Formulas and Equations Box
Essential Calculations:
- Concentration: C = n/V or C = m/(Mr × V)
- Moles: n = m/Mr or n = C × V
- Titration: n₁V₁ = n₂V₂
- Percentage purity: (Actual yield/Theoretical yield) × 100
- Atom economy: (Mr of desired product/Sum of Mr of all products) × 100
Key Reactions:
- Metal + acid → salt + hydrogen
- Carbonate + acid → salt + water + carbon dioxide
- Hydroxide + acid → salt + water
Quick Revision Notes
Qualitative Analysis Quick Check:
✓ Flame tests show metal ions by color
✓ Hydroxide precipitates identify metal ions
✓ Gas tests use characteristic reactions
✓ Anion tests use specific reagents
Quantitative Analysis Quick Check:
✓ Titration finds unknown concentrations
✓ Indicators show end points
✓ Calculations use mole ratios
✓ Accurate measurement is crucial
Laboratory Techniques Quick Check:
✓ Read burettes at eye level
✓ Rinse all apparatus properly
✓ Use appropriate separation methods
✓ Follow safety procedures always
Common Mistakes to Avoid
1. Reading the Meniscus Wrong
- Always read at eye level
- Read to the bottom of the meniscus for water-based solutions
2. Contaminating Solutions
- Always rinse apparatus with the solution being used
- Never return unused chemicals to original bottles
3. Rushing Titrations
- Add titrant drop by drop near the end point
- Swirl constantly for proper mixing
4. Ignoring Significant Figures
- Match your answer to the least precise measurement
- Burette readings: 2 decimal places
- Pipette volumes: usually 1 decimal place
Test Yourself Questions
Quick Quiz:
- What color flame does potassium produce?
- Which indicator would you use for a strong acid-weak base titration?
- How do you test for carbon dioxide gas?
- What precipitate forms when silver nitrate is added to chloride ions?
- Calculate the concentration if 0.05 moles are dissolved in 250 cm³ of solution.
Answers:
- Lilac/violet
- Methyl orange
- Turns limewater milky
- White precipitate of silver chloride
- 0.2 mol/dm³
Important Exam-Style Questions
1. Describe how you would identify an unknown metal ion using flame tests and hydroxide precipitation. [6 marks]
2. A student titrated 25.0 cm³ of 0.1 mol/dm³ sodium hydroxide with hydrochloric acid. 23.5 cm³ of acid was needed. Calculate the concentration of the hydrochloric acid. [4 marks]
3. Explain why distilled water is used to rinse apparatus and why this doesn’t affect the results. [3 marks]
Real-World Applications
Environmental Monitoring:
- Testing water quality in rivers and lakes
- Measuring air pollution levels
- Analyzing soil composition for agriculture
Food Industry:
- Checking vitamin content in supplements
- Measuring acidity in wines and juices
- Testing for food preservatives
Medical Field:
- Blood glucose level testing
- Drug purity analysis
- Mineral deficiency diagnosis
Remember, experimental chemistry is a skill that improves with practice. Every professional chemist started exactly where you are now. The techniques you’re learning aren’t just for exams – they’re the foundation of scientific discovery and innovation.
Whether you’re planning to pursue chemistry further or just want to ace your IGCSE, mastering these experimental techniques opens doors to understanding how our world works at the molecular level. From developing life-saving medicines to creating sustainable materials, these skills are your gateway to making a real difference.
Keep practicing, stay curious, and remember – every expert was once a beginner. You’ve got this!
Good luck with your IGCSE Chemistry journey – the world of experimental chemistry awaits your discovery!
Recommended –