The Amazing World of Chemical Reactions
Imagine baking a cake, watching fireworks explode in the sky, or even breathing – all these everyday activities involve chemical reactions! Chemical reactions are happening around us every second, transforming one substance into another. In IGCSE Chemistry Topic 6, we’ll explore this fascinating world where atoms dance, bonds break and form, and energy flows like an invisible river.
Whether you’re struggling with balancing equations or wondering why some reactions happen faster than others, this guide will transform your understanding from confusion to confidence. Let’s embark on this chemical journey together!
What Are Chemical Reactions? The Foundation
A chemical reaction is a process where one or more substances (reactants) are converted into different substances (products). Think of it like a recipe – you mix ingredients (reactants) following specific conditions, and you get something completely new (products).
The key difference between physical and chemical changes:
- Physical change: Ice melting to water (same substance, different state)
- Chemical change: Wood burning to ash and carbon dioxide (completely different substances)
Signs That a Chemical Reaction Has Occurred:
- Color change (copper turning green when exposed to air)
- Gas production (bubbles forming when you add baking soda to vinegar)
- Temperature change (hand warmers getting hot)
- Precipitate formation (solid forming in a liquid mixture)
- Light emission (glow sticks or fireworks)
Types of Chemical Reactions: The Big Five
Understanding reaction types is like learning different dance moves – once you know the patterns, you can recognize them anywhere!
1. Synthesis (Combination) Reactions
Pattern: A + B → AB
Two or more simple substances combine to form a more complex compound.
Example: 2H₂ + O₂ → 2H₂O
Hydrogen and oxygen gases combine to form water
Real-life application: Formation of rust (iron + oxygen → iron oxide)
2. Decomposition Reactions
Pattern: AB → A + B
A complex compound breaks down into simpler substances.
Example: 2H₂O → 2H₂ + O₂
Water breaks down into hydrogen and oxygen gases (electrolysis)
Memory tip: “Decomposition = Breaking Apart” – think of leaves decomposing in autumn!
3. Single Displacement (Replacement) Reactions
Pattern: A + BC → AC + B
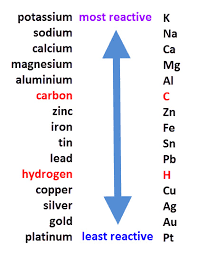
A more reactive element replaces a less reactive element in a compound.
Example: Zn + CuSO₄ → ZnSO₄ + Cu
Zinc displaces copper from copper sulfate
Key concept: Check the reactivity series! More reactive metals displace less reactive ones.
4. Double Displacement (Precipitation) Reactions
Pattern: AB + CD → AD + CB
Two compounds exchange ions to form two new compounds.
Example: AgNO₃ + NaCl → AgCl + NaNO₃
Silver nitrate and sodium chloride form silver chloride precipitate and sodium nitrate
5. Combustion Reactions
Pattern: Fuel + O₂ → CO₂ + H₂O + Energy
A substance burns in oxygen, releasing energy.
Complete combustion example: CH₄ + 2O₂ → CO₂ + 2H₂O
Methane burns completely to form carbon dioxide and water
Incomplete combustion example: 2CH₄ + 3O₂ → 2CO + 4H₂O
Limited oxygen supply produces carbon monoxide instead
Chemical Equations: The Language of Chemistry
Chemical equations are like sentences in chemistry – they tell the complete story of what happens in a reaction.
Writing and Balancing Chemical Equations
Step-by-step approach:
- Write the word equation first
Magnesium + Oxygen → Magnesium oxide - Convert to chemical formula
Mg + O₂ → MgO - Balance the equation
2Mg + O₂ → 2MgO - Check your work
Left side: 2 Mg atoms, 2 O atoms
Right side: 2 Mg atoms, 2 O atoms ✓
Memory trick for balancing: “Balance like a seesaw – what goes on one side must equal the other!”
State Symbols: The Reaction’s Environment
- (s) = solid
- (l) = liquid
- (g) = gas
- (aq) = aqueous (dissolved in water)
Example: NaCl(s) + H₂O(l) → Na⁺(aq) + Cl⁻(aq)
Energy Changes in Chemical Reactions
Every chemical reaction involves energy changes – some release energy (making you feel warm), while others absorb energy (making things feel cold).
Exothermic Reactions: The Energy Givers
Definition: Reactions that release energy to the surroundings
Characteristics:
- Temperature of surroundings increases
- Energy is given out (usually as heat or light)
- Products have less energy than reactants
Examples:
- Combustion (burning wood)
- Respiration (glucose + oxygen → energy + CO₂ + H₂O)
- Neutralization (acid + base → salt + water + heat)
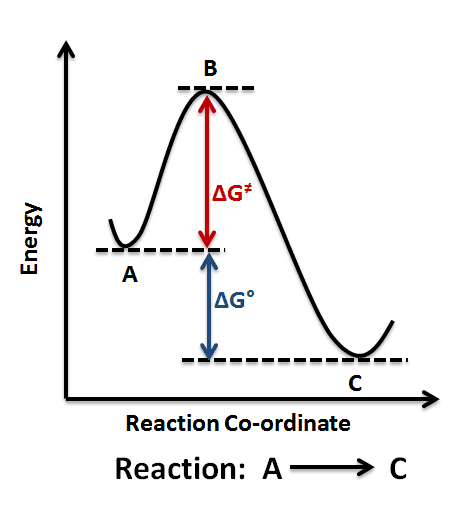
Energy diagram: Products are lower than reactants
Endothermic Reactions: The Energy Takers
Definition: Reactions that absorb energy from the surroundings
Characteristics:
- Temperature of surroundings decreases
- Energy is taken in (usually as heat)
- Products have more energy than reactants
Examples:
- Photosynthesis (CO₂ + H₂O + light energy → glucose + O₂)
- Thermal decomposition (heating calcium carbonate)
- Melting ice (physical change example)
Energy diagram: Products are higher than reactants
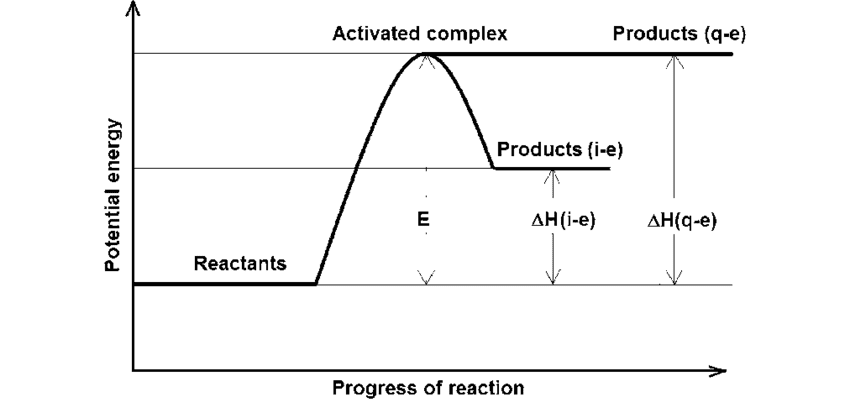
Activation Energy: The Energy Barrier
Think of activation energy as the “push” needed to start a reaction – like pushing a boulder over a hill.
Definition: The minimum energy required for a reaction to occur
Key points:
- All reactions need activation energy to start
- Higher activation energy = slower reaction
- Catalysts lower activation energy
- Temperature affects how many particles have enough energy to react
Analogy: Starting a car – you need to turn the key (activation energy) before the engine runs (reaction proceeds)
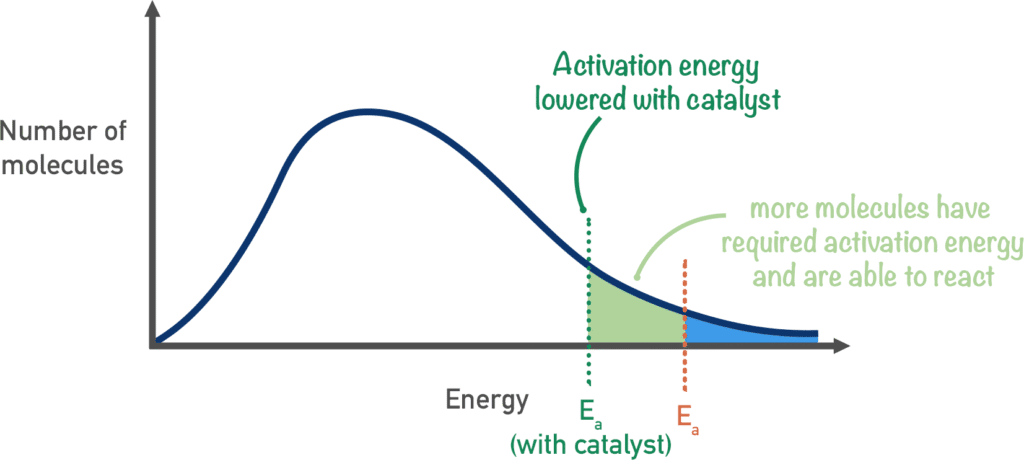
Factors Affecting Reaction Rates
Understanding what makes reactions faster or slower is crucial for controlling chemical processes.
1. Temperature
Rule: Higher temperature = faster reaction
Explanation:
- Particles move faster at higher temperatures
- More collisions occur per second
- More particles have energy ≥ activation energy
Everyday example: Food cooks faster at higher oven temperatures
2. Concentration/Pressure
Rule: Higher concentration = faster reaction
Explanation:
- More particles in the same space
- More frequent collisions
- Higher probability of successful collisions
Laboratory example: Concentrated hydrochloric acid reacts faster with zinc than dilute acid
3. Surface Area
Rule: Larger surface area = faster reaction
Explanation:
- More particles exposed to react
- Powder reacts faster than lumps
- Breaking solids into smaller pieces increases surface area
Practical example: Sugar dissolves faster when stirred (increases surface area contact)
4. Catalysts
Definition: Substances that speed up reactions without being consumed
How they work:
- Provide alternative reaction pathway
- Lower activation energy
- Same amount of catalyst present at start and end
Types:
- Homogeneous catalysts: Same phase as reactants
- Heterogeneous catalysts: Different phase from reactants
Examples:
- Enzymes in biological reactions
- Platinum in catalytic converters
- Iron in Haber process (ammonia production)
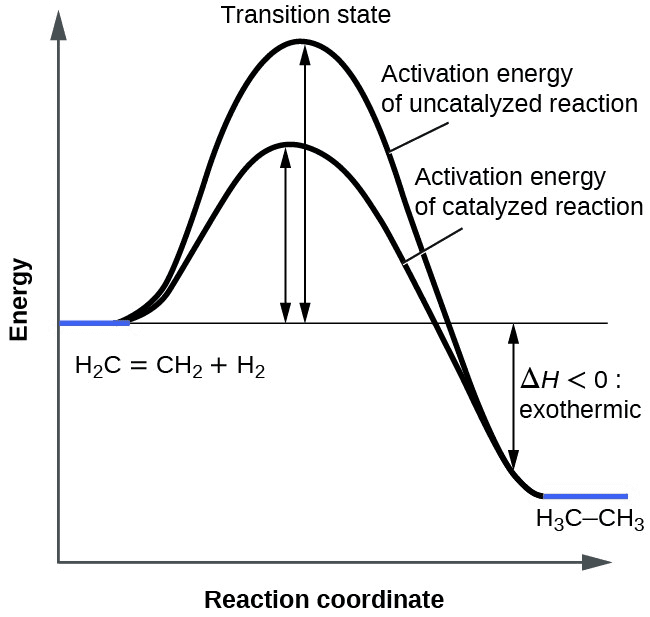
Collision Theory: Why Reactions Happen
For a reaction to occur, particles must:
- Collide with each other
- Have sufficient energy (≥ activation energy)
- Collide with correct orientation
Effective collision: Results in bond breaking and forming
Ineffective collision: Particles bounce off without reacting
Factors increasing effective collisions:
- Higher temperature (more energetic particles)
- Higher concentration (more particles to collide)
- Larger surface area (more collision opportunities)
- Presence of catalyst (lower energy requirement)
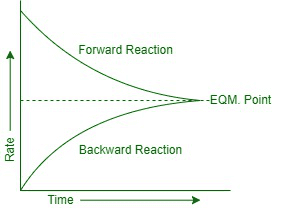
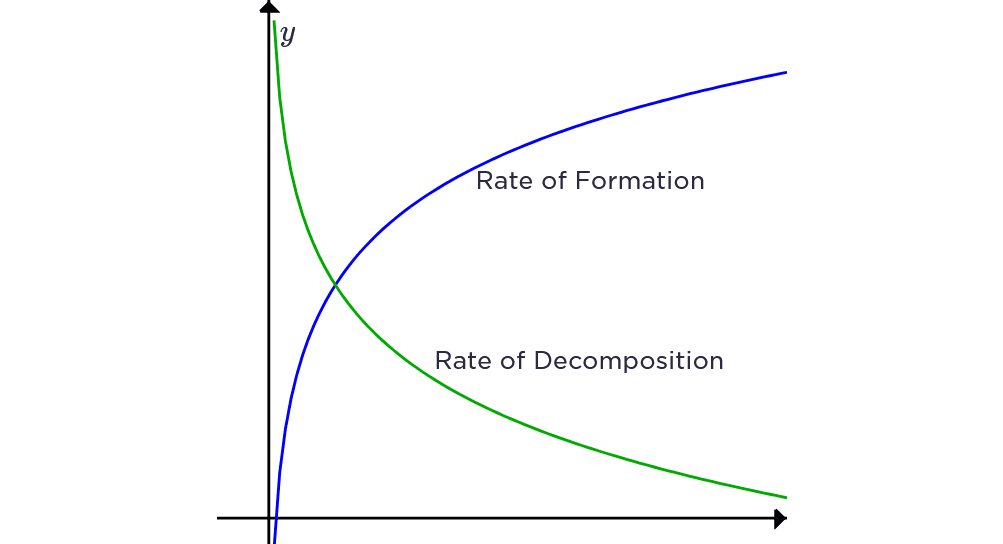
Reversible Reactions and Equilibrium
Some reactions can go both ways – like a revolving door that can spin in either direction!
Reversible reaction notation: A + B ⇌ C + D
Dynamic equilibrium:
- Forward and reverse reaction rates are equal
- Concentrations of reactants and products remain constant
- Reactions continue in both directions
Le Chatelier’s Principle: If conditions change, the equilibrium shifts to oppose that change
Factors affecting equilibrium:
- Temperature change: Equilibrium shifts to counteract temperature change
- Pressure change: Equilibrium shifts toward side with fewer gas molecules
- Concentration change: Equilibrium shifts away from added substance
Industrial example: Haber process for ammonia production
N₂(g) + 3H₂(g) ⇌ 2NH₃(g) + heat
Important Chemical Equations for IGCSE
Key Formulas and Equations Box:
Combustion Reactions:
- Methane: CH₄ + 2O₂ → CO₂ + 2H₂O
- Ethane: 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
Acid-Base Reactions:
- HCl + NaOH → NaCl + H₂O
- H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O
Metal-Acid Reactions:
- Zn + 2HCl → ZnCl₂ + H₂
- Mg + H₂SO₄ → MgSO₄ + H₂
Thermal Decomposition:
- CaCO₃ → CaO + CO₂
- 2Cu(NO₃)₂ → 2CuO + 4NO₂ + O₂
Precipitation Reactions:
- AgNO₃ + NaCl → AgCl + NaNO₃
- BaCl₂ + Na₂SO₄ → BaSO₄ + 2NaCl
Common Mistakes to Avoid
1. Balancing Equations Incorrectly
Wrong: H₂ + O₂ → H₂O₂
Correct: 2H₂ + O₂ → 2H₂O
Tip: Never change chemical formulas – only add coefficients!
2. Confusing Exothermic and Endothermic
Memory trick:
- EXothermic = EXit energy (energy leaves the system)
- ENdothermic = ENter energy (energy enters the system)
3. Forgetting State Symbols
Always include (s), (l), (g), or (aq) in equations when required
4. Misunderstanding Catalysts
Remember: Catalysts speed up reactions but are NOT consumed in the process
Test Yourself: Quick Practice Questions
- What type of reaction is: 2Na + Cl₂ → 2NaCl?
- Will increasing temperature speed up ALL reactions?
- What happens to activation energy when a catalyst is added?
- Give two signs that a chemical reaction has occurred.
- Balance: Al + O₂ → Al₂O₃
Answers:
- Synthesis/Combination reaction
- Yes, higher temperature increases reaction rates
- Activation energy decreases
- Any two: color change, gas production, temperature change, precipitate formation, light emission
- 4Al + 3O₂ → 2Al₂O₃
Quick Revision Notes
Reaction Types Summary:
- Synthesis: A + B → AB
- Decomposition: AB → A + B
- Single displacement: A + BC → AC + B
- Double displacement: AB + CD → AD + CB
- Combustion: Fuel + O₂ → CO₂ + H₂O + Energy
Energy Changes:
- Exothermic: Releases energy, products lower energy than reactants
- Endothermic: Absorbs energy, products higher energy than reactants
Rate Factors (Temperature, Concentration, Surface Area, Catalysts):
- Increase any factor → Increase reaction rate
- Catalysts lower activation energy but aren’t consumed
Collision Theory Requirements:
- Particles must collide
- Sufficient energy (≥ activation energy)
- Correct orientation
Exam Success Tips
For Equation Balancing:
- Start with the most complex molecule
- Balance metals first, then non-metals, then hydrogen and oxygen
- Use fractions if needed, then multiply through to get whole numbers
- Always double-check your work
For Energy Diagrams:
- Exothermic: Arrow pointing down from reactants to products
- Endothermic: Arrow pointing up from reactants to products
- Always label activation energy peak
- Show energy released/absorbed
Common Exam Questions Types:
- Identify reaction type from given equation
- Balance chemical equations
- Explain energy changes using particle theory
- Describe factors affecting reaction rates
- Draw and interpret energy diagrams
- Explain how catalysts work
Important Exam Questions
Long Answer Questions:
Question 1: Explain why increasing temperature increases the rate of reaction. Use collision theory in your answer. (6 marks)
Model Answer Structure:
- Define collision theory
- Explain how temperature affects particle movement
- Link to activation energy concept
- Mention frequency and energy of collisions
- Conclude with overall effect on reaction rate
Question 2: Draw an energy diagram for an exothermic reaction. Label the activation energy, reactants, products, and energy released. (5 marks)
Question 3: Explain the difference between a catalyst and increasing temperature as methods of speeding up reactions. (4 marks)
Calculation Questions:
Question 4: If 2.4g of magnesium reacts completely with hydrochloric acid, calculate the volume of hydrogen gas produced at room temperature and pressure. (Given: Mg = 24, molar volume = 24 dm³)
Equation: Mg + 2HCl → MgCl₂ + H₂
Real-World Applications
Industrial Processes:
- Haber Process: Manufacturing ammonia for fertilizers
- Contact Process: Producing sulfuric acid
- Blast Furnace: Extracting iron from iron ore
Everyday Examples:
- Cooking: Maillard reactions creating flavors and colors
- Cleaning: Enzyme detergents breaking down stains
- Medicine: Controlled drug release using reaction rates
Environmental Applications:
- Catalytic converters: Reducing car emissions
- Photosynthesis: Converting CO₂ to oxygen
- Combustion: Energy production and pollution
Study Strategy for Success
Memory Techniques:
- Acronym for reaction types: “Some Dogs Sit Down Calmly” (Synthesis, Decomposition, Single displacement, Double displacement, Combustion)
- Energy changes: “EX-out, EN-in” (Exothermic releases, Endothermic absorbs)
Practice Resources:
- Past paper questions (2019-2024)
- Cambridge textbook exercises
- Online simulation tools for collision theory
- Practical experiment observations
Conclusion: Your Chemical Reaction Journey
Congratulations! You’ve just completed a comprehensive journey through IGCSE Chemistry Topic 6. Chemical reactions aren’t just academic concepts – they’re the fundamental processes that power our world, from the food we digest to the technology we use.
Remember, chemistry is like learning a new language. At first, the symbols and equations might seem overwhelming, but with practice, you’ll start thinking like a chemist. Every balanced equation you write, every energy diagram you draw, and every reaction type you identify brings you closer to understanding the incredible world of chemical transformations.
Your Next Steps:
- Practice regularly – Solve at least 5 equation balancing problems daily
- Connect to real life – Notice chemical reactions happening around you
- Teach others – Explaining concepts to friends reinforces your understanding
- Stay curious – Ask “why” and “how” when studying reactions
Related Topics to Explore:
- Topic 7: Chemical energetics (building on energy changes)
- Topic 8: Acids, bases and salts (applying reaction types)
- Topic 9: The Periodic Table (understanding reactivity patterns)
Remember: Every expert was once a beginner. Every mistake is a learning opportunity. Every practice question solved is a step toward exam success.
You’ve got this! Chemical reactions are waiting to reveal their secrets to you. Keep practicing, stay curious, and watch your understanding transform from confusion to confidence, just like the amazing chemical reactions you’re now equipped to understand.
Final encouragement: The same determination that helps you balance a tricky equation will serve you well in all areas of life. Chemistry teaches us that with the right conditions, energy, and persistence, any transformation is possible – including your transformation into a confident chemistry student!
Good luck with your studies, and may your passion for chemistry continue to react and grow!
Recommended –

1 thought on “IGCSE (Cambridge) Chemistry Topic 6: Chemical Reactions | Your Complete Study Guide”