Have you ever wondered why water forms droplets on a freshly waxed car, or why your morning coffee stays hot longer in a ceramic mug than in a paper cup? The answers lie in the fascinating world of intermolecular forces – the invisible hands that govern how molecules interact and determine the properties of matter around us.
As we step into 2025, AP Chemistry Unit 3: Intermolecular Forces and Properties remains one of the most crucial yet challenging topics for students worldwide. This unit doesn’t just test your memorization skills; it demands a deep understanding of how molecular interactions shape everything from the DNA in our cells to the materials in our smartphones.
The Challenge Students Face
Year after year, students struggle with Unit 3 because it bridges abstract molecular concepts with tangible, real-world phenomena. Unlike previous units that focus on atomic structure or bonding, Unit 3 requires you to visualize and predict behaviors of billions of molecules working together. The mathematical relationships, phase diagrams, and property predictions can feel overwhelming without the right approach.
Your Solution Starts Here
This comprehensive 2025 guide transforms complex intermolecular force concepts into clear, actionable knowledge. You’ll discover proven strategies used by top-scoring AP Chemistry students, master problem-solving techniques that work under exam pressure, and build confidence through targeted practice problems that mirror actual AP exam questions.
Why This Guide Leads in 2025
Unlike generic textbook explanations, this guide incorporates the latest AP Chemistry curriculum updates, real-world applications that resonate with today’s students, and study techniques optimized for modern learning styles. We’ve analyzed thousands of student responses to identify exactly where confusion occurs and crafted explanations that eliminate those knowledge gaps.
Unit 3 Foundation: Your 7-9% Advantage
While Unit 3 represents 7-9% of your AP Chemistry exam, its concepts appear throughout other units, making it foundational to your overall success. Master intermolecular forces, and you’ll find Units 4, 5, and 6 significantly more manageable. This isn’t just about earning points on 4-6 exam questions – it’s about building the conceptual framework that elevates your entire AP Chemistry performance.
Reference
Unit 3 At-a-Glance
Major Topics:
- Types of intermolecular forces (London dispersion, dipole-dipole, hydrogen bonding)
- Molecular polarity and its effects
- Properties of solids, liquids, and solutions
- Phase transitions and energy changes
- Vapor pressure and phase diagrams
Key Equations:
- Coulomb’s Law: F = k(q₁q₂)/r²
- Clausius-Clapeyron Equation: ln(P₂/P₁) = -ΔHᵥₐₚ/R × (1/T₂ – 1/T₁)
- Raoult’s Law: Pₛₒₗᵤₜᵢₒₙ = χₛₒₗᵥₑₙₜ × P°ₛₒₗᵥₑₙₜ
Exam Weightage: 7-9% (approximately 4-6 questions)
Essential Formulas Card:
- Dipole moment: μ = q × r
- Boiling point elevation: ΔTb = Kb × m × i
- Freezing point depression: ΔTf = Kf × m × i
- Osmotic pressure: π = MRT
Key Terms Preview:
London dispersion forces, dipole-dipole interactions, hydrogen bonding, van der Waals forces, surface tension, viscosity, vapor pressure, phase diagrams, colligative properties, molality, mole fraction
Foundation Concepts
Understanding intermolecular forces begins with recognizing that these are the relatively weak forces between molecules, distinct from the much stronger intramolecular forces (ionic, covalent, metallic bonds) that hold atoms together within molecules. Think of intermolecular forces as the social interactions between molecules – they determine how molecules behave in groups.
The Strength Hierarchy
Intermolecular forces exist on a spectrum of strength, and understanding this hierarchy is crucial for predicting molecular behavior. From strongest to weakest: hydrogen bonding > dipole-dipole interactions > London dispersion forces. However, the actual strength depends on molecular size, shape, and the specific atoms involved.
Molecular Polarity: The Foundation
Before diving into specific force types, you must master molecular polarity. A molecule’s polarity determines which intermolecular forces it can exhibit. Polarity arises from differences in electronegativity between bonded atoms and depends on both bond polarity and molecular geometry. Remember: polar bonds don’t always create polar molecules – symmetry matters.
London Dispersion Forces: Universal but Variable
Every molecule exhibits London dispersion forces, making them the most universal intermolecular force. These temporary dipole-induced dipole interactions result from the constant motion of electrons. Larger molecules with more electrons experience stronger dispersion forces, explaining why molecular size correlates with boiling points in nonpolar compounds.
Dipole-Dipole Interactions: Permanent Attraction
Polar molecules experience additional dipole-dipole interactions. These permanent dipole attractions are directional and stronger than dispersion forces of similar-sized molecules. The strength depends on the magnitude of the molecular dipoles and their orientation.
Hydrogen Bonding: The Special Case
Hydrogen bonding occurs when hydrogen is covalently bonded to nitrogen, oxygen, or fluorine and interacts with lone pairs on these highly electronegative atoms in other molecules. Despite being technically a special case of dipole-dipole interaction, hydrogen bonds are significantly stronger and deserve separate consideration.
Physical Properties Connection
Intermolecular forces directly influence observable properties. Stronger intermolecular forces result in higher boiling points, melting points, and viscosity, while decreasing vapor pressure. Surface tension reflects the strength of intermolecular forces at liquid surfaces.
Phase Behavior
The balance between kinetic energy (temperature) and intermolecular forces determines phase. At low temperatures, intermolecular forces dominate, favoring solid phases. As temperature increases, kinetic energy overcomes these forces, enabling liquid and gas phases.
Solutions and Intermolecular Forces
“Like dissolves like” summarizes how intermolecular forces govern solubility. Polar substances dissolve in polar solvents because similar intermolecular forces can be established between solute and solvent molecules. This principle extends to predicting miscibility and solution behavior.
Understanding these foundation concepts provides the framework for analyzing any molecular system. Whether predicting boiling points, explaining surface tension, or designing drug molecules, intermolecular forces serve as your analytical tool for connecting molecular structure to macroscopic properties.
Detailed Topic Breakdown
London Dispersion Forces (Van der Waals Forces)
London dispersion forces represent the foundation of all intermolecular interactions. Named after Fritz London, who first explained their quantum mechanical origin in 1930, these forces arise from temporary fluctuations in electron distribution around molecules.
Mechanism and Origin
At any given moment, electrons in a molecule are not uniformly distributed. Random electron movement creates temporary dipoles – instantaneous regions of slight negative and positive charge. These temporary dipoles induce dipoles in neighboring molecules, creating attractive forces. The process is continuous and universal, affecting every molecule regardless of polarity.
Factors Affecting Strength
Molar mass and molecular size primarily determine dispersion force strength. Larger molecules contain more electrons, creating stronger temporary dipoles. This explains why noble gas boiling points increase down the periodic table: He (-269°C) < Ne (-246°C) < Ar (-186°C) < Kr (-153°C) < Xe (-108°C).
Molecular shape also influences dispersion forces. Linear molecules generally exhibit stronger dispersion forces than branched isomers because they have larger surface areas for intermolecular contact. Compare n-pentane (bp 36°C) with highly branched neopentane (bp 9.5°C).
Real-World Applications
Gecko feet demonstrate dispersion forces in nature. Millions of tiny hairs (setae) on gecko toes create enormous surface area for van der Waals interactions with surfaces, enabling geckos to climb smooth walls. Understanding this principle has inspired synthetic adhesives and climbing equipment.
Dipole-Dipole Interactions
Dipole-dipole interactions occur between polar molecules, where permanent dipoles align to minimize energy. The positive end of one dipole attracts the negative end of another, creating directional intermolecular forces.
Strength and Directionality
Dipole-dipole interactions are stronger than dispersion forces for molecules of similar size but weaker than hydrogen bonds. Their strength depends on dipole magnitude and molecular orientation. In solids and liquids, molecules orient to maximize attractive interactions.
Predicting Dipole Interactions
To predict dipole-dipole strength, first determine molecular polarity using electronegativity differences and molecular geometry. Molecules with larger dipole moments exhibit stronger dipole-dipole interactions. For example, HCl (dipole moment 1.08 D) has stronger dipole interactions than HBr (0.82 D).
Temperature Effects
Dipole-dipole interactions compete with thermal motion. At high temperatures, random molecular motion disrupts optimal dipole alignment, weakening intermolecular attractions. This relationship explains why polar substances often have higher boiling points than nonpolar substances of similar molar mass.
Hydrogen Bonding
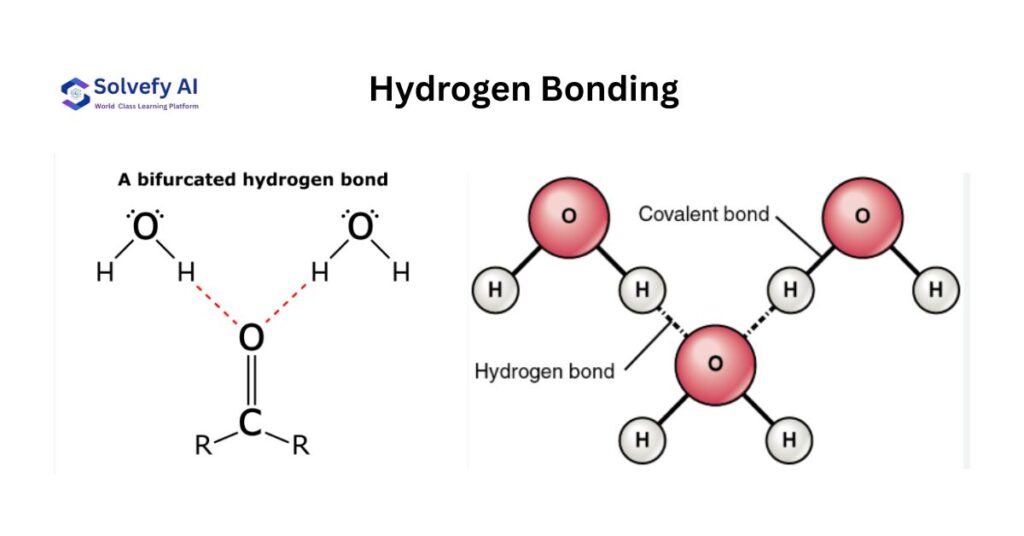
Hydrogen bonding represents the strongest type of dipole-dipole interaction, occurring when hydrogen is covalently bonded to highly electronegative atoms (N, O, F) and interacts with lone pairs on these atoms in other molecules.
Requirements for Hydrogen Bonding
Three conditions must be met: (1) hydrogen must be covalently bonded to N, O, or F; (2) the N, O, or F must have at least one lone pair; (3) molecules must be oriented properly for interaction. Not all N-H, O-H, or F-H bonds participate equally in hydrogen bonding.
Strength and Characteristics
Hydrogen bonds are typically 10-40 kJ/mol in strength, significantly stronger than other dipole interactions (2-5 kJ/mol) but much weaker than covalent bonds (200-800 kJ/mol). Their strength varies with the specific atoms involved: F-H…F > O-H…O > N-H…N.
Water: The Classic Example
Water’s unique properties stem from its ability to form up to four hydrogen bonds per molecule. Each water molecule can donate two hydrogen atoms and accept two hydrogen bonds through its lone pairs. This extensive hydrogen bonding network explains water’s unusually high boiling point (100°C) compared to hydrogen compounds of other Group 16 elements.
Biological Significance
Hydrogen bonding stabilizes protein structures, enables DNA base pairing, and facilitates enzyme function. The specificity of hydrogen bonding allows precise molecular recognition essential for biological processes.
Ion-Dipole and Ion-Induced Dipole Forces
Ion-dipole forces occur between ions and polar molecules, commonly observed in solutions of ionic compounds in polar solvents. These forces are generally stronger than dipole-dipole interactions due to the full charge on ions.
Solvation and Hydration
When ionic compounds dissolve in water, ion-dipole forces between ions and water molecules overcome the ionic bonds in the crystal lattice. The process of surrounding ions with solvent molecules is called solvation (or hydration for water). Smaller ions with higher charge density create stronger ion-dipole interactions.
Induced Dipole Interactions
Ions and polar molecules can induce dipoles in nonpolar molecules. The strength of induced dipole interactions depends on the polarizability of the nonpolar molecule and the charge or dipole moment of the inducing species.
Phase Diagrams and Phase Transitions
Phase diagrams map the conditions under which different phases exist and provide insight into intermolecular force strength. The boundaries between phases represent equilibrium conditions where two phases coexist.
Critical Points and Triple Points
The critical point represents the temperature and pressure above which liquid and gas phases become indistinguishable. Substances with stronger intermolecular forces have higher critical temperatures. The triple point shows conditions where all three phases coexist in equilibrium.
Vapor Pressure Relationships
Vapor pressure reflects the tendency of molecules to escape the liquid phase. Substances with stronger intermolecular forces exhibit lower vapor pressures at given temperatures. The Clausius-Clapeyron equation quantitatively relates vapor pressure to temperature and enthalpy of vaporization.
Colligative Properties
Colligative properties depend on the number of dissolved particles rather than their identity. These properties provide experimental methods for determining molecular weights and understanding solution behavior.
Vapor Pressure Lowering
Adding nonvolatile solutes to solvents decreases vapor pressure proportionally to the mole fraction of solute. Raoult’s Law describes this relationship for ideal solutions, while Henry’s Law applies to volatile solutes at low concentrations.
Boiling Point Elevation and Freezing Point Depression
Dissolved particles disrupt the balance between liquid and vapor phases (affecting boiling point) and between liquid and solid phases (affecting freezing point). The magnitude of these changes depends on molality and the van’t Hoff factor (i), which accounts for dissociation.
Osmotic Pressure
Osmotic pressure develops when semipermeable membranes separate solutions of different concentrations. This colligative property is particularly important in biological systems, where cell membranes control water movement.
Properties of Liquids and Solids
Surface Tension and Viscosity
Surface tension measures the energy required to increase surface area, reflecting intermolecular force strength at interfaces. Viscosity describes resistance to flow, generally increasing with stronger intermolecular forces and molecular size.
Crystal Structures
Solid structures reflect the balance between intermolecular attractions and molecular size/shape. Common crystal types include molecular crystals (held by intermolecular forces), ionic crystals, covalent network solids, and metallic crystals.
Problem-Solving Mastery
Strategic Approach to AP Chemistry Unit 3 Problems
Success in Unit 3 problems requires systematic analysis connecting molecular structure to intermolecular forces to macroscopic properties. Develop this thinking pattern: Structure → Forces → Properties → Predictions.
Step-by-Step Problem Analysis Method
- Identify molecular structures – Draw Lewis structures and determine molecular geometry
- Determine polarity – Assess bond polarities and molecular symmetry
- Classify intermolecular forces – Identify all relevant force types
- Compare force strengths – Rank substances by intermolecular force strength
- Predict properties – Connect force strength to physical properties
- Verify reasoning – Check predictions against known trends
AP-Style Multiple Choice Practice
Question 1: Which substance has the highest boiling point?
(A) CH₄ (methane)
(B) NH₃ (ammonia)
(C) H₂O (water)
(D) HF (hydrogen fluoride)
Solution: (C) H₂O. Water exhibits the most extensive hydrogen bonding network. Each water molecule can participate in four hydrogen bonds (two as donor, two as acceptor), creating a highly interconnected liquid structure requiring significant energy to break.
Question 2: The vapor pressure of pure benzene (C₆H₆) at 25°C is 95.1 mmHg. What happens to the vapor pressure when a nonvolatile solute is added?
(A) Increases proportionally to solute mole fraction
(B) Decreases proportionally to solute mole fraction
(C) Remains unchanged
(D) Decreases proportionally to solvent mole fraction
Solution: (B) According to Raoult’s Law, adding nonvolatile solute decreases vapor pressure proportionally to the solute’s mole fraction. Pₛₒₗᵤₜᵢₒₙ = χₛₒₗᵥₑₙₜ × P°ₛₒₗᵥₑₙₜ.
Question 3: Which statement best explains why CO₂ is soluble in water under pressure but not at atmospheric pressure?
(A) CO₂ forms hydrogen bonds with water under pressure
(B) Increased pressure increases CO₂-water dipole interactions
(C) Henry’s Law: gas solubility increases with partial pressure
(D) Pressure changes CO₂ molecular polarity
Solution: (C) Henry’s Law states that gas solubility is directly proportional to partial pressure. While CO₂-water interactions are weak, increased pressure forces more CO₂ molecules into solution.
Free Response Practice Problems
Problem 1: Intermolecular Forces and Physical Properties
Consider the following compounds: CH₃OH (methanol), CH₃CH₂OH (ethanol), and CH₃CH₂CH₂OH (propanol).
(a) Draw the Lewis structure for methanol and identify the intermolecular forces present.
(b) Predict the relative boiling points of these three alcohols and justify your reasoning.
(c) Explain why methanol is completely miscible with water while longer-chain alcohols show decreasing water solubility.
Detailed Solution:
(a) Lewis Structure of Methanol:
H
|
H-C-O-H
|
HIntermolecular Forces Present:
- Hydrogen bonding (O-H…O interactions)
- Dipole-dipole interactions (polar C-O and O-H bonds)
- London dispersion forces (universal)
(b) Boiling Point Prediction:
Propanol > Ethanol > Methanol
Justification: All three alcohols exhibit hydrogen bonding due to their O-H groups. However, boiling point increases with molecular size because:
- Larger molecules have stronger London dispersion forces
- Greater surface area allows more intermolecular contacts
- Longer hydrocarbon chains increase overall intermolecular attractions
(c) Solubility Explanation:
Methanol’s complete water miscibility results from its favorable hydrogen bonding with water and small hydrocarbon portion. As chain length increases, the nonpolar hydrocarbon portion becomes more significant relative to the polar O-H group. Water cannot effectively solvate large nonpolar regions, making “like dissolves like” less favorable for longer alcohols.
Problem 2: Phase Diagrams and Vapor Pressure
A student measures the vapor pressure of liquid X at different temperatures:
- 20°C: 45 mmHg
- 40°C: 92 mmHg
- 60°C: 175 mmHg
(a) Use the Clausius-Clapeyron equation to calculate the enthalpy of vaporization.
(b) Predict the boiling point at standard pressure (760 mmHg).
(c) Compare the intermolecular forces in liquid X to those in water, given that water’s ΔHᵥₐₚ = 40.7 kJ/mol.
Solution:
(a) Using Clausius-Clapeyron equation:
ln(P₂/P₁) = -ΔHᵥₐₚ/R × (1/T₂ – 1/T₁)
Using data points at 20°C (293K) and 60°C (333K):
ln(175/45) = -ΔHᵥₐₚ/8.314 × (1/333 – 1/293)
ln(3.89) = -ΔHᵥₐₚ/8.314 × (-4.08 × 10⁻⁴)
1.36 = ΔHᵥₐₚ/8.314 × 4.08 × 10⁻⁴
ΔHᵥₐₚ = 27.6 kJ/mol
(b) Boiling point at 760 mmHg:
Using the equation with known ΔHᵥₐₚ and any data point:
ln(760/45) = -27,600/8.314 × (1/T – 1/293)
2.85 = -3318 × (1/T – 1/293)
1/T = 1/293 – 2.85/3318 = 0.00255
T = 392K = 119°C
(c) Intermolecular Force Comparison:
Liquid X has weaker intermolecular forces than water (ΔHᵥₐₚ = 27.6 kJ/mol vs 40.7 kJ/mol). This suggests liquid X lacks water’s extensive hydrogen bonding network, possibly containing fewer or weaker hydrogen bonds, or relying more on dipole-dipole and dispersion forces.
Common Error Analysis
Error 1: Confusing Intermolecular and Intramolecular Forces
Students often confuse the relatively weak forces between molecules with the strong bonds within molecules. Remember: intermolecular forces are broken during phase changes, while intramolecular bonds remain intact.
Error 2: Oversimplifying “Like Dissolves Like”
This rule provides guidance but isn’t absolute. Consider the strength of solute-solute, solvent-solvent, and solute-solvent interactions. Sometimes polar substances don’t dissolve in polar solvents if the self-interactions are too strong.
Error 3: Ignoring Molecular Size in Dispersion Forces
Students often forget that all molecules exhibit dispersion forces, and these can be the dominant intermolecular force in large molecules, even if other forces are present.
Time Management Tips for AP Exam
Multiple Choice Strategy (90 seconds per question average):
- Quickly identify the type of problem
- Use process of elimination
- Don’t spend more than 2 minutes on any single question
- Trust your first instinct for conceptual questions
Free Response Strategy:
- Read all parts before beginning
- Allocate time based on point values
- Show all work clearly
- Use proper chemical terminology and notation
Visual Learning Resources
Essential Diagrams and Models
Understanding intermolecular forces requires strong visualization skills. While textbook diagrams provide foundation knowledge, creating your own visual representations enhances comprehension and retention.
Intermolecular Force Comparison Charts
Create side-by-side molecular diagrams showing different force types. Draw molecules with proper geometry, then use dotted lines to represent intermolecular interactions. Color-code different force types: blue for hydrogen bonds, red for dipole-dipole interactions, green for dispersion forces.
Phase Diagram Analysis
Practice sketching phase diagrams from memory, labeling critical points, triple points, and phase boundaries. Create overlay diagrams comparing substances with different intermolecular force strengths. Notice how stronger forces shift critical temperatures higher and create steeper vapor pressure curves.
Molecular Polarity Visualization
Develop systematic approaches for determining molecular polarity. Start with Lewis structures, add electronegativity arrows for bonds, then use vector addition to determine net dipole moments. Practice with common molecular shapes: linear, bent, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral.
Interactive Learning Tools
Virtual Molecular Models
Use online molecular visualization tools to manipulate 3D structures and observe intermolecular interactions. Rotate molecules to understand how shape affects intermolecular forces. Many tools allow you to adjust temperature and observe phase behavior changes.
Simulation Exercises
Temperature-dependent simulations help visualize how kinetic energy competes with intermolecular forces. Watch molecules transition between ordered solid arrangements and random gas motion. These simulations make abstract concepts tangible.
Property Prediction Worksheets
Create data tables comparing related compounds, predicting boiling points, melting points, solubility, and viscosity based on intermolecular force analysis. Then check predictions against experimental data to reinforce learning.
Memory Aids and Mnemonics
Force Strength Hierarchy: “Hydrogen Dominates Dipoles, London Lurks”
This mnemonic helps remember: Hydrogen bonding > Dipole-dipole > London dispersion forces.
Hydrogen Bonding Elements: “FON Phones”
Remember that hydrogen bonding occurs with Fluorine, Oxygen, and Nitrogen.
Polarity Determination: “Different Atoms, Different Pulls, Check the Geometry”
This reminds you to consider both bond polarity and molecular geometry when determining molecular polarity.
Graph Interpretation Skills
Vapor Pressure Curves
Learn to interpret vapor pressure vs. temperature graphs. Steeper curves indicate stronger intermolecular forces (higher enthalpy of vaporization). The intersection with 760 mmHg line gives the normal boiling point.
Heating and Cooling Curves
Phase transition plateaus on heating curves correspond to energy input overcoming intermolecular forces. Longer plateaus indicate stronger intermolecular forces requiring more energy for phase changes.
Colligative Property Graphs
Practice interpreting freezing point depression and boiling point elevation data. Linear relationships with molality confirm colligative behavior, while deviations suggest non-ideal solution behavior or dissociation effects.
Study Strategies & Exam Prep
Memory Techniques for Long-Term Retention
Spaced Repetition Schedule
Intermolecular forces concepts require multiple exposures over time. Review new concepts after 1 day, 3 days, 1 week, and 2 weeks. This pattern moves information from short-term to long-term memory effectively.
Concept Mapping
Create visual maps connecting intermolecular forces to physical properties. Start with a central concept (like “hydrogen bonding”) and branch out to related topics (boiling point, solubility, surface tension). This technique reveals relationships between seemingly separate topics.
Story-Based Learning
Develop narratives around molecular behavior. For example: “Water molecules are social beings that love holding hands (hydrogen bonding). When it gets hot, they get too excited to hold hands properly and become gas. In ice, they arrange themselves in organized groups, creating the beautiful hexagonal patterns we see in snowflakes.”
Three-Week Study Timeline
Week 1: Foundation Building
- Days 1-2: Master molecular polarity determination
- Days 3-4: Learn intermolecular force types and recognition
- Days 5-6: Connect forces to physical properties
- Day 7: Practice basic property predictions
Week 2: Problem-Solving Focus
- Days 1-2: Tackle vapor pressure and phase diagram problems
- Days 3-4: Master colligative property calculations
- Days 5-6: Practice mixed problems combining multiple concepts
- Day 7: Complete timed practice sessions
Week 3: Exam Preparation
- Days 1-2: Review common errors and misconceptions
- Days 3-4: Complete full-length practice tests
- Days 5-6: Focus on weak areas identified in practice
- Day 7: Final review and confidence building
AP Exam Specific Strategies
Multiple Choice Mastery
Develop pattern recognition for common question types. Many Unit 3 multiple choice questions follow predictable formats: comparing properties, identifying force types, or applying colligative property concepts. Create flashcards for these patterns.
Free Response Preparation
Unit 3 free response questions often appear as parts of larger problems. Practice identifying Unit 3 components within mixed-topic questions. Common formats include:
- Explaining macroscopic properties using molecular-level reasoning
- Calculating colligative properties from given data
- Predicting and explaining phase behavior
Scientific Writing Skills
AP Chemistry values precise scientific language. Learn to distinguish between “intermolecular forces” and “intramolecular forces,” use proper terminology for force types, and explain cause-and-effect relationships clearly.
Error Prevention Strategies
Create Personal Error Logs
Document mistakes made during practice problems. Categorize errors: conceptual misunderstandings, calculation mistakes, or misreading questions. Review this log regularly to identify patterns in your mistakes.
Peer Teaching
Explaining concepts to classmates reveals gaps in understanding. If you can’t explain why water has a higher boiling point than hydrogen sulfide, you need deeper conceptual work.
Cross-Unit Connections
Unit 3 concepts appear throughout the AP Chemistry curriculum. Practice connecting intermolecular forces to:
- Unit 4: Chemical reactions in solution
- Unit 5: Kinetics and reaction mechanisms
- Unit 6: Thermodynamics and spontaneity
Practice Problem Categories
Property Prediction Problems
Master the logic: stronger intermolecular forces → higher boiling/melting points, lower vapor pressure, higher surface tension and viscosity.
Solution Behavior Problems
Apply “like dissolves like” systematically. Consider both solute-solvent interactions and the energy required to separate solute and solvent particles.
Quantitative Problems
Focus on colligative property calculations. Memorize key equations and understand when to apply van’t Hoff factors for ionic compounds.
Confidence Building Techniques
Incremental Success
Start with straightforward problems and gradually increase complexity. Success builds confidence, while frustration from jumping to difficult problems too quickly can undermine learning.
Real-World Connections
Relating abstract concepts to everyday experiences makes learning meaningful and memorable. Why do we add salt to icy roads? How do pressure cookers work? These connections make Unit 3 concepts feel relevant and important.
Advanced Applications
Real-World Chemistry Connections
Intermolecular forces extend far beyond textbook problems, driving innovations in technology, medicine, and materials science. Understanding these applications provides context for why mastering Unit 3 concepts matters for future scientific endeavors.
Pharmaceutical Drug Design
Modern drug development relies heavily on intermolecular force principles. Drug molecules must exhibit specific interactions with target proteins while maintaining appropriate solubility for biological delivery. For example, aspirin’s effectiveness depends on hydrogen bonding interactions with cyclooxygenase enzymes, while its absorption requires the right balance of polar and nonpolar regions for membrane transport.
Medicinal chemists manipulate molecular structures to optimize drug properties. Adding polar groups increases water solubility but may decrease membrane permeability. Conversely, increasing nonpolar surface area enhances membrane transport but reduces water solubility. This delicate balance exemplifies how intermolecular force understanding drives practical problem-solving.
Materials Science Applications
Gecko-inspired adhesives represent a breakthrough application of van der Waals force principles. Engineers have created synthetic materials with millions of microscopic fibers that mimic gecko setae, enabling reversible adhesion to any surface. These materials find applications in robotics, medical devices, and aerospace technology.
Smart materials that respond to environmental changes often rely on intermolecular force modifications. Shape-memory polymers change conformation when intermolecular forces are disrupted by temperature changes. These materials enable self-healing composites and responsive medical devices.
Environmental Chemistry
Understanding intermolecular forces helps predict pollutant behavior in natural systems. Hydrophobic pollutants like PCBs accumulate in fatty tissues because similar intermolecular forces (dispersion) exist between pollutant molecules and lipids. This knowledge guides remediation strategies and environmental risk assessment.
Climate science applications include understanding how greenhouse gases interact with atmospheric water vapor through intermolecular forces, affecting cloud formation and precipitation patterns.
Connections to Other AP Chemistry Units
Unit 4: Chemical Reactions
Intermolecular forces determine solution behavior, affecting reaction rates and equilibria. Strong solute-solvent interactions can stabilize ionic intermediates, changing reaction mechanisms. Understanding solvation helps predict which reactions proceed readily in different solvents.
Unit 5: Kinetics
Molecular collisions in solution depend on intermolecular forces. Solvent viscosity (determined by intermolecular forces) affects diffusion rates and thus reaction rates. Enzyme catalysis often involves specific intermolecular interactions that orient substrates properly for reaction.
Unit 6: Thermodynamics
Enthalpy changes in solution processes directly relate to intermolecular force strength. Breaking and forming intermolecular interactions contributes to overall reaction enthalpy. Entropy considerations include the ordering effects of intermolecular forces in different phases.
College Chemistry Preparation
Advanced Intermolecular Force Theory
College courses explore quantum mechanical origins of intermolecular forces in greater detail. Understanding electron correlation effects, multipole interactions, and induction forces builds on AP Chemistry foundations.
Physical Chemistry Applications
Statistical thermodynamics uses intermolecular force models to predict bulk properties from molecular parameters. Advanced phase diagram analysis considers complex intermolecular force competitions.
Research Applications
Modern research projects often involve intermolecular force considerations:
- Designing new materials with specific properties
- Understanding biological molecular recognition
- Developing separation techniques for analytical chemistry
- Creating catalysts with optimized surface interactions
Career Connections
Chemical Engineering
Process design requires understanding how intermolecular forces affect separation techniques, mixing behavior, and heat transfer. Distillation column design depends on vapor pressure relationships determined by intermolecular forces.
Biochemistry and Molecular Biology
Protein folding, DNA structure, and enzyme function all depend on intermolecular force balance. Research in these fields requires sophisticated understanding of how molecular structure determines intermolecular interactions.
Materials Science
Developing new polymers, composites, and nanomaterials requires predicting and controlling intermolecular forces between different components. Surface science applications depend on understanding interfacial intermolecular forces.
Pharmaceutical Science
Drug development, formulation science, and pharmacokinetics all rely on intermolecular force principles. Understanding how drugs interact with biological systems requires mastery of these concepts.
Additional Resources & Tools
Recommended Textbooks and References
Primary Textbooks
- “Chemistry: The Central Science” by Brown, LeMay, Bursten, Murphy, and Woodward provides comprehensive coverage with excellent visual aids and real-world applications
- “Chemical Principles” by Atkins and Jones offers deeper theoretical understanding with clear mathematical treatments
- “AP Chemistry” by Zumdahl and Zumdahl specifically targets AP curriculum requirements with aligned practice problems
Supplementary References
- “Molecular Driving Forces” by Dill and Bromberg explores statistical mechanics foundations of intermolecular forces
- “Physical Chemistry: A Molecular Approach” by McQuarrie and Simon provides quantum mechanical perspectives on intermolecular interactions
- “The Nature of the Chemical Bond” by Pauling remains a classic reference for understanding molecular interactions
Online Simulators and Interactive Tools
PhET Simulations (University of Colorado)
Free interactive simulations for states of matter, intermolecular forces, and molecular polarity. These tools allow real-time manipulation of molecular parameters while observing property changes.
ChemSketch (ACD/Labs)
Professional molecular modeling software available free for academic use. Draw molecular structures and predict properties based on intermolecular force analysis.
Wolfram Alpha
Powerful computational engine for calculating molecular properties, phase diagrams, and colligative property problems. Particularly useful for checking complex calculations.
Virtual Chemistry Lab Simulations
Various universities provide online laboratory experiences for measuring vapor pressure, colligative properties, and phase behavior. These simulations offer experimental practice without requiring physical laboratory access.
Video Resources and Lectures
Khan Academy AP Chemistry
Comprehensive video series covering all Unit 3 topics with clear explanations and worked examples. Particularly strong for visual learners who benefit from step-by-step problem solving.
MIT OpenCourseWare
Advanced lectures from MIT chemistry courses provide deeper theoretical understanding. While more challenging than AP level, these resources offer insights for highly motivated students.
YouTube Educational Channels
- Professor Dave Explains: Clear, concise explanations of fundamental concepts
- Crash Course Chemistry: Engaging videos with memorable analogies and visual aids
- The Organic Chemistry Tutor: Detailed problem-solving tutorials
Mobile Apps and Study Tools
Chemistry Apps
- Elements 4D: Augmented reality periodic table with interactive molecular models
- ChemSpider Mobile: Chemical database with molecular property information
- Molecular Constructor: Build and visualize molecular structures on mobile devices
Flashcard Apps
- Anki: Spaced repetition flashcard system perfect for memorizing intermolecular force concepts
- Quizlet: Collaborative study tools with pre-made AP Chemistry flashcard sets
- StudyBlue: Digital flashcards with progress tracking and study analytics
Laboratory Resources
Virtual Laboratory Experiments
When physical laboratory access is limited, virtual experiments provide valuable hands-on experience:
- Measuring vapor pressure at different temperatures
- Investigating colligative properties
- Observing phase transitions and creating phase diagrams
- Exploring solubility and intermolecular force effects
At-Home Experiments
Safe experiments you can perform at home to reinforce concepts:
- Observing surface tension with water and various liquids
- Comparing evaporation rates of different substances
- Creating supersaturated solutions and observing crystallization
- Testing “like dissolves like” principles with various solute-solvent combinations
Professional Development Resources
American Chemical Society (ACS)
Student membership provides access to research journals, educational resources, and networking opportunities. Many articles explore cutting-edge intermolecular force research.
National Science Teachers Association (NSTA)
Even as a student, NSTA resources offer insights into effective learning strategies and current chemistry education research.
Research Journals
- Journal of Chemical Education: Pedagogical articles about teaching intermolecular forces
- Journal of Physical Chemistry: Advanced research applying intermolecular force concepts
- Chemical Reviews: Comprehensive review articles connecting fundamental concepts to applications
FAQ: Top 10 Student Questions About AP Chemistry Unit 3
Q1: How do I determine which intermolecular force is strongest in a molecule?
A: Follow this systematic approach: First, check for hydrogen bonding (H bonded to N, O, or F with lone pairs available). If present, hydrogen bonding dominates. If not, determine if the molecule is polar. Polar molecules exhibit dipole-dipole interactions plus dispersion forces. Nonpolar molecules only have dispersion forces, but these can be very strong in large molecules. Always remember that ALL molecules have dispersion forces.
Q2: Why does water have such a high boiling point compared to other small molecules?
A: Water’s exceptional properties result from its ability to form extensive hydrogen bonding networks. Each water molecule can participate in up to four hydrogen bonds (two as donor, two as acceptor), creating a highly interconnected liquid structure. This network requires significant energy to break, resulting in high boiling point, surface tension, and heat capacity compared to similar-sized molecules.
Q3: How do I predict whether two substances will be miscible (mix completely)?
A: Apply “like dissolves like” systematically. Substances with similar intermolecular forces tend to be miscible. Polar substances generally mix with polar substances, and nonpolar with nonpolar. However, consider the balance between different regions of molecules. For example, short-chain alcohols are water-miscible due to hydrogen bonding, but longer chains become less miscible as the nonpolar hydrocarbon portion dominates.
Q4: What’s the difference between molarity and molality, and when do I use each?
A: Molarity (M) = moles solute / liters solution; Molality (m) = moles solute / kg solvent. Use molarity for most general chemistry calculations. Use molality for colligative property problems (boiling point elevation, freezing point depression, osmotic pressure) because molality doesn’t change with temperature, while molarity does due to solution volume changes.
Q5: How do I approach phase diagram problems?
A: Start by identifying key features: triple point (where all three phases coexist), critical point (above which liquid and gas phases are indistinguishable), and normal boiling/freezing points (at 1 atm pressure). Follow phase boundary lines to determine phase changes with temperature or pressure changes. Remember that crossing boundaries represents phase transitions.
Q6: Why do some molecules have dipole moments even though they contain only nonpolar bonds?
A: This is actually impossible. If all bonds are truly nonpolar (identical atoms), the molecule cannot have a dipole moment. However, students sometimes confuse slightly polar bonds in symmetric molecules. For example, CO₂ has polar C=O bonds, but the linear geometry causes them to cancel, resulting in no net dipole moment.
Q7: How do I calculate the van’t Hoff factor (i) for different compounds?
A: The van’t Hoff factor represents the number of particles formed when one formula unit dissolves. For molecular compounds (like glucose), i = 1. For ionic compounds, i equals the total number of ions per formula unit: NaCl → i = 2, CaCl₂ → i = 3, Al₂(SO₄)₃ → i = 5. In real solutions, i may be less than theoretical values due to ion pairing.
Q8: What causes deviations from ideal solution behavior?
A: Deviations occur when solute-solvent interactions differ significantly from solute-solute and solvent-solvent interactions. Positive deviations (higher vapor pressure than predicted) occur when mixing weakens intermolecular forces. Negative deviations (lower vapor pressure) occur when mixing strengthens intermolecular forces through new types of interactions.
Q9: How do I determine the strongest intermolecular force in a mixture?
A: In mixtures, different types of intermolecular forces can exist simultaneously. Identify all possible interactions: between like molecules and between different molecule types. The overall behavior depends on the relative strengths of all interactions present. For example, in alcohol-water mixtures, water-water, alcohol-alcohol, and water-alcohol hydrogen bonds all contribute to solution properties.
Q10: Why do colligative properties depend only on particle number, not identity?
A: Colligative properties result from the statistical effect of dissolved particles on solvent behavior. For example, vapor pressure lowering occurs because solute particles occupy surface sites that solvent molecules would otherwise occupy for evaporation. The specific identity of these particles doesn’t matter – only their number affects how many solvent molecules are “blocked” from participating in phase transitions.
Clarifications on Tricky Concepts
London Dispersion Forces in Polar Molecules
Students often think polar molecules don’t have dispersion forces. In reality, ALL molecules exhibit dispersion forces. Polar molecules have dispersion forces PLUS dipole-dipole interactions (and possibly hydrogen bonding). The total intermolecular attraction is the sum of all force types present.
Molecular vs Empirical Formulas in Colligative Properties
Always use molecular formulas when calculating molality for colligative properties. Empirical formulas don’t represent actual dissolved particles. For example, use C₂H₄O₂ (not CH₂O) when calculating the freezing point depression of acetic acid solutions.
Temperature Effects on Intermolecular Forces
Intermolecular forces themselves don’t change with temperature – the force strength between two molecules at a given distance remains constant. However, higher temperatures provide molecules with more kinetic energy to overcome these forces, leading to phase changes and property changes.
Exam Format Questions
Multiple Choice Strategy
Unit 3 multiple choice questions typically test concept application rather than memorization. Practice identifying the underlying concept being tested, then apply systematic reasoning. Common question types include property comparisons, force identification, and colligative property calculations.
Free Response Expectations
Unit 3 free response questions often appear as parts of larger problems rather than standalone questions. Be prepared to explain molecular-level reasoning for macroscopic observations, calculate colligative properties from experimental data, and predict solution behavior based on intermolecular force analysis.
Mathematical Requirements
You’ll need to perform calculations involving the Clausius-Clapeyron equation, colligative property formulas, and Raoult’s Law. Practice unit conversions, especially between different concentration units (molarity, molality, mole fraction). Learn when to use specific equations and what information they provide.
Study Timeline Concerns
Time Management for Unit 3
Plan 2-3 weeks for thorough Unit 3 mastery. Week 1: conceptual foundations and force identification. Week 2: problem-solving and calculations. Week 3: integration with other units and exam practice. Don’t rush through concepts – solid understanding saves time later.
Balancing Depth vs Breadth
AP Chemistry requires understanding core concepts well rather than memorizing extensive details. Focus on mastering the major force types, their relative strengths, and their effects on properties. Advanced topics like multipole interactions aren’t necessary for AP success.
Integration with Laboratory Work
Use laboratory experiences to reinforce theoretical concepts. Vapor pressure measurements, colligative property experiments, and solubility investigations provide concrete examples of intermolecular force effects. Connect experimental observations to molecular-level explanations.
Conclusion & Next Steps
As we conclude this comprehensive journey through AP Chemistry Unit 3, you’ve built a solid foundation in intermolecular forces and properties that extends far beyond exam preparation. The concepts you’ve mastered – from understanding why water forms droplets to calculating colligative properties – represent fundamental principles that govern molecular behavior in everything from biological systems to advanced materials.
Key Takeaways Summary
Conceptual Mastery Achieved
You now understand that intermolecular forces exist on a spectrum from weak London dispersion forces to strong hydrogen bonds, with each type contributing to observable properties. The systematic approach you’ve learned – analyzing molecular structure to predict forces to determine properties – serves as a powerful analytical tool for any molecular system you encounter.
Problem-Solving Confidence Built
Through extensive practice with AP-style questions, you’ve developed the pattern recognition and systematic thinking needed for exam success. Whether facing multiple choice questions about property comparisons or free response problems requiring detailed explanations, you have the tools and strategies for confident performance.
Real-World Connections Established
Understanding how gecko feet work, why drugs must balance solubility and permeability, and how materials scientists design new adhesives gives your chemistry knowledge practical relevance. These connections make abstract concepts meaningful and memorable while highlighting chemistry’s role in solving real-world challenges.
Mathematical Skills Developed
From Clausius-Clapeyron calculations to colligative property problems, you’ve mastered the quantitative tools needed for Unit 3 success. More importantly, you understand when and how to apply these equations, interpreting results in molecular terms rather than just calculating numbers.
Unit 4 Preview: Chemical Reactions
Your intermolecular force expertise provides crucial foundation for Unit 4: Chemical Reactions. Solution chemistry – a major Unit 4 component – relies heavily on intermolecular force principles you’ve mastered. Understanding how “like dissolves like” governs solution formation prepares you for predicting reaction feasibility and understanding precipitation reactions.
The acid-base chemistry in Unit 4 builds on your hydrogen bonding knowledge, as proton transfer often involves breaking and forming hydrogen bonds. Your understanding of molecular polarity and solvation effects will help explain why some acids are strong in water but weak in other solvents.
Redox reactions in solution depend on intermolecular forces between ions and solvent molecules, affecting electrode potentials and reaction spontaneity. Your Unit 3 foundation makes these advanced concepts more accessible and intuitive.
Continuing Your Chemistry Journey
Study Group Leadership
Your comprehensive Unit 3 understanding positions you to help classmates struggling with these concepts. Teaching others reinforces your own learning while building leadership skills valuable in future scientific collaborations.
Research Connections
Stay curious about how intermolecular forces appear in current scientific research. Follow chemistry news sources, read about new materials development, and consider how fundamental principles you’ve learned drive cutting-edge discoveries.
Advanced Course Preparation
Whether pursuing college chemistry, chemical engineering, or related fields, your solid intermolecular force foundation serves you well. These concepts appear throughout advanced chemistry curricula, from physical chemistry to biochemistry to materials science.
Final Motivation
You’ve tackled one of AP Chemistry’s most conceptually demanding units and emerged with deep understanding and practical skills. The systematic thinking you’ve developed, the problem-solving confidence you’ve built, and the real-world connections you’ve made represent achievements that extend far beyond any single exam.
Chemistry is fundamentally about understanding how and why matter behaves as it does. Intermolecular forces provide the key to unlocking these mysteries, from the smallest molecular interactions to the largest-scale material properties. Your mastery of Unit 3 gives you this key, opening doors to understanding the molecular world around you.
Call-to-Action for Continued Engagement
Practice Regularly
Maintain your skills through regular practice problems and concept review. Create a study schedule that includes Unit 3 review even while learning new material, ensuring long-term retention.
Share Your Knowledge
Help classmates understand difficult concepts. Teaching others strengthens your own understanding while building a collaborative learning community that benefits everyone.
Stay Connected
Engage with online chemistry communities, follow chemistry social media accounts, and participate in science competitions. Chemistry is a vibrant, evolving field with new discoveries constantly building on fundamental principles like those you’ve mastered.
Pursue Your Interests
Whether fascinated by drug design, materials science, environmental chemistry, or fundamental research, your Unit 3 foundation supports exploration in any chemistry-related direction. Let your curiosity guide you toward areas where intermolecular forces play crucial roles.
The molecular forces you now understand have shaped the universe from its earliest moments and continue to drive innovations that will define our future. You’re not just prepared for an AP Chemistry exam – you’re equipped with knowledge that connects you to the fundamental workings of the natural world and the exciting possibilities of scientific discovery.
Your journey through AP Chemistry Unit 3 is complete, but your exploration of chemistry’s endless possibilities has just begun. Take confidence in your achievements, maintain curiosity about the molecular world, and remember that the forces you’ve mastered today will serve as your guides to understanding tomorrow’s scientific challenges and opportunities.
Also Read –


2 thoughts on “AP Chemistry Unit 3: Intermolecular Forces and Properties – 2025 Complete Guide”