Introduction: The Metal World Around Us
Take a moment to look around you right now. That smartphone in your hand? It contains precious metals like gold and silver. The car outside your window? Built from steel, an iron alloy. The coins in your pocket? Made from copper and nickel alloys. Even the water pipes in your home rely on metals to function properly.
Welcome to Topic 9 of IGCSE Chemistry – arguably one of the most practical and fascinating topics in your entire syllabus! This isn’t just about memorizing facts; it’s about understanding the materials that literally built our modern world.
In this comprehensive guide, we’ll explore everything from why some metals are found as pure nuggets in nature while others need complex extraction processes, to why your bicycle doesn’t rust but the old car in your neighbor’s yard does. By the end of this journey, you’ll not only master the concepts for your IGCSE Chemistry 0620 exam but also gain a deeper appreciation for the incredible world of metals.
What Makes Metals So Special?
Physical Properties of Metals
Before diving into the complex chemistry, let’s establish what makes a metal… well, a metal! Metals possess a unique combination of properties that make them incredibly useful:
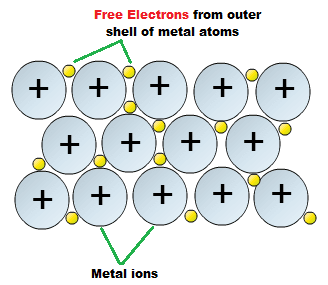
Conductivity Champions: Metals are excellent conductors of both heat and electricity. This happens because of their unique electron structure – they have ‘free electrons’ that can move around easily. Think of it like a crowd of people where everyone can pass messages quickly from person to person.
Malleable and Ductile: You can hammer metals into thin sheets (malleability) or stretch them into wires (ductility) without breaking them. This is why we can make everything from aluminum foil to copper wires.
Lustrous and Strong: Metals have that characteristic shiny appearance when polished, and most are mechanically strong. However, strength varies dramatically – compare soft sodium (you can cut it with a knife) with hard tungsten (used in drill bits).
High Density and Melting Points: Most metals are dense and have high melting points, though there are notable exceptions like mercury, which is liquid at room temperature.
Chemical Properties: The Reactivity Story
Here’s where things get really interesting! Not all metals behave the same way chemically. Some metals like gold can sit in the ground for thousands of years without changing, while others like sodium react so violently with water that they can explode.
This difference in chemical behavior is what we call reactivity, and it’s the key to understanding everything else in this topic.
The Reactivity Series: Your Metal Hierarchy
Understanding Metal Reactivity
The reactivity series is like a league table for metals – it ranks them from most reactive (potassium at the top) to least reactive (gold at the bottom). This isn’t just academic knowledge; it’s practical information that explains why we use certain metals for specific purposes.
The Complete Reactivity Series (from most to least reactive):
- Potassium (K) – Extremely reactive
- Sodium (Na) – Very reactive
- Lithium (Li) – Very reactive
- Calcium (Ca) – Reactive
- Magnesium (Mg) – Reactive
- Aluminum (Al) – Moderately reactive
- Carbon (C) – Non-metal reference point
- Zinc (Zn) – Moderately reactive
- Iron (Fe) – Less reactive
- Tin (Sn) – Less reactive
- Lead (Pb) – Less reactive
- Hydrogen (H) – Non-metal reference point
- Copper (Cu) – Unreactive
- Silver (Ag) – Very unreactive
- Gold (Au) – Extremely unreactive
Memory Tip: “Please Send Lions, Cats, Monkeys And Cute Zebras Into The Lead House Carrying Silver Guns”
Experimental Evidence for Reactivity
The reactivity series isn’t just theoretical – it’s based on experimental observations. Here’s how we can determine the order:
1. Reaction with Water:
- Potassium and sodium react explosively with cold water
- Calcium reacts vigorously with cold water
- Magnesium reacts slowly with cold water but rapidly with steam
- Aluminum, zinc, and iron react with steam only
- Metals below hydrogen don’t react with water at all
2. Reaction with Acids:
- Metals above hydrogen in the series react with acids to produce hydrogen gas
- The more reactive the metal, the more vigorous the reaction
- Metals below hydrogen cannot displace hydrogen from acids
3. Displacement Reactions:
- A more reactive metal can displace a less reactive metal from its compound
- Example: Zinc displaces copper from copper sulfate solution
Zn + CuSO₄ → ZnSO₄ + Cu
Real-World Applications of Reactivity
Understanding reactivity explains many everyday phenomena:
Why Gold Doesn’t Tarnish: Gold is so unreactive that it doesn’t combine with oxygen or sulfur compounds in the air, maintaining its brilliant shine for centuries.
Why Iron Rusts: Iron is reactive enough to combine with oxygen and water, forming rust (hydrated iron oxide).
Why Aluminum Doesn’t Seem to Rust: Aluminum actually reacts with oxygen very quickly, but it forms a thin, protective layer of aluminum oxide that prevents further reaction.
Metal Extraction: From Rocks to Resources
The Challenge of Metal Extraction
Most metals don’t exist as pure elements in nature – they’re locked up in compounds called ores. An ore is a naturally occurring rock that contains enough of a metal compound to make extraction economically worthwhile.
The key question is: How do we get the pure metal out of its compound? The answer depends entirely on where the metal sits in the reactivity series.
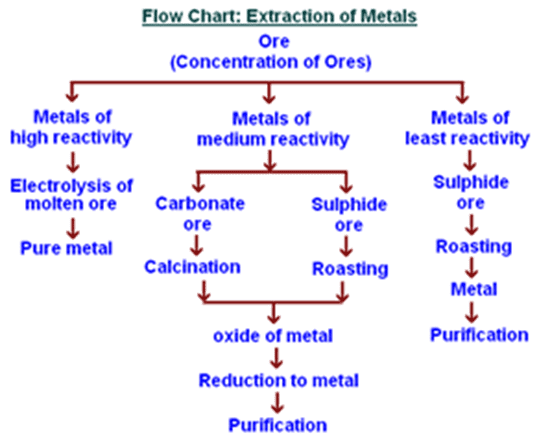
Extraction Methods Based on Reactivity
1. For Very Unreactive Metals (Gold, Silver):
These metals are sometimes found as pure elements in nature because they’re so unreactive. When extraction is needed, simple physical methods or mild chemical treatments work.
2. For Moderately Reactive Metals (Iron, Zinc, Lead):
These metals are extracted using carbon reduction. Since carbon sits in the middle of our reactivity series, it can displace metals that are less reactive than itself.
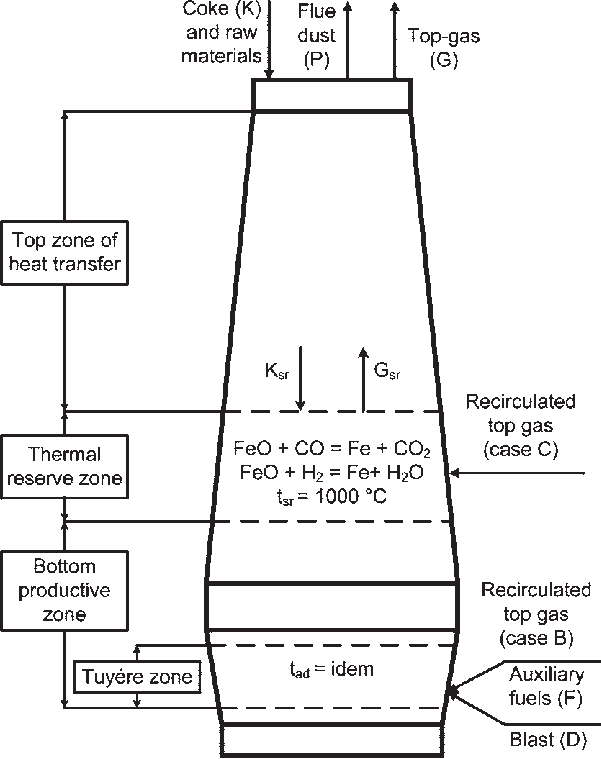
The Iron Extraction Process (Blast Furnace):
This is probably the most important industrial process you’ll study:
Raw Materials:
- Iron ore (hematite, Fe₂O₃)
- Coke (carbon)
- Limestone (calcium carbonate, CaCO₃)
Key Reactions:
- C + O₂ → CO₂ (carbon burns in air)
- CO₂ + C → 2CO (carbon dioxide reacts with more carbon)
- Fe₂O₃ + 3CO → 2Fe + 3CO₂ (iron oxide is reduced)
- CaCO₃ → CaO + CO₂ (limestone decomposes)
- CaO + SiO₂ → CaSiO₃ (limestone removes impurities as slag)
3. For Very Reactive Metals (Aluminum, Magnesium):
These metals are above carbon in the reactivity series, so carbon cannot displace them. Instead, we use electrolysis – using electricity to force the reaction.
Aluminum Extraction (Hall-Héroult Process):
- Aluminum oxide (Al₂O₃) is dissolved in molten cryolite
- Electricity passes through the molten mixture
- At the cathode: Al³⁺ + 3e⁻ → Al
- At the anode: 2O²⁻ → O₂ + 4e⁻
This process requires enormous amounts of electricity, which is why aluminum was once more expensive than gold!
Why Different Methods for Different Metals?
The extraction method depends on the stability of the metal compound:
- Very reactive metals form very stable compounds that need a lot of energy (electricity) to break apart
- Moderately reactive metals form moderately stable compounds that can be broken apart by carbon (a chemical reducing agent)
- Unreactive metals form unstable compounds or exist as pure elements
Alloys: Making Metals Even Better
What Are Alloys?
Pure metals, while useful, often have limitations. They might be too soft, too reactive, or lack specific properties needed for particular applications. This is where alloys come in – alloys are mixtures of metals, or metals with non-metals, designed to have improved properties.
Think of alloys as “designer metals” – we mix different elements to create materials with exactly the properties we need.
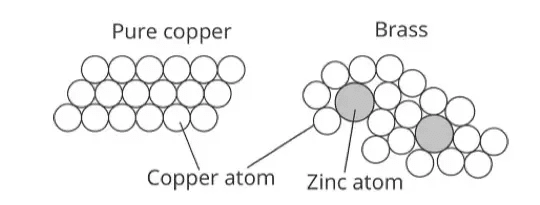
How Alloys Work
The secret lies in the structure. In pure metals, all atoms are the same size and arranged in regular patterns. When we add different elements:
- Different sized atoms disrupt the regular arrangement
- This makes it harder for layers of atoms to slide over each other
- Result: The alloy becomes harder and stronger than pure metals
Common Alloys and Their Applications
Steel (Iron + Carbon):
- Pure iron is actually quite soft and rusts easily
- Adding small amounts of carbon (0.1-2%) creates steel
- Different carbon percentages create different types:
- Low carbon steel: car bodies, construction
- High carbon steel: cutting tools, springs
- Stainless steel (+ chromium): cutlery, medical instruments
Bronze (Copper + Tin):
- One of humanity’s first alloys (Bronze Age!)
- Harder than both copper and tin individually
- Uses: statues, coins, bearings
Brass (Copper + Zinc):
- Golden color, doesn’t rust
- Uses: musical instruments, door handles, decorative items
Duralumin (Aluminum + Copper + Magnesium):
- Light like aluminum but much stronger
- Uses: aircraft construction, spacecraft
Why We Don’t Always Use Pure Metals
Pure Gold Problems:
- 24-karat gold is too soft for jewelry
- 18-karat gold (75% gold + 25% other metals) is much more durable
Pure Aluminum Limitations:
- Soft and not strong enough for structural uses
- Aluminum alloys are used in everything from soda cans to airplanes
Corrosion: The Enemy of Metals
Understanding Corrosion
Corrosion is essentially the reverse of metal extraction – metals naturally want to return to their compound form. It’s the destruction of materials by chemical reactions with substances in the environment.
The most familiar example is rusting, but corrosion affects many metals in different ways.
The Science of Rusting
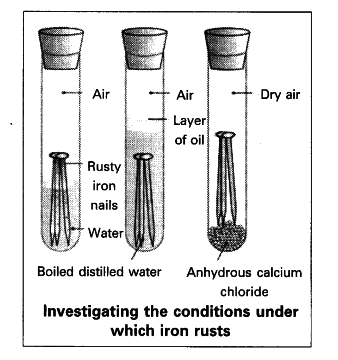
Rusting is the corrosion of iron and steel. For rusting to occur, you need three things:
- Iron (obviously!)
- Oxygen from the air
- Water (as liquid or water vapor)
The Rusting Process:
- Iron atoms lose electrons: Fe → Fe²⁺ + 2e⁻
- Oxygen gains electrons: O₂ + 4H⁺ + 4e⁻ → 2H₂O
- Iron(II) is further oxidized: Fe²⁺ → Fe³⁺ + e⁻
- Hydrated iron(III) oxide forms: Fe₂O₃.xH₂O (rust)
Experimental Evidence:
- Boiled water (no oxygen): no rusting
- Dry air (no water): no rusting
- Calcium chloride (removes water): no rusting
- Both water and oxygen present: rapid rusting
Preventing Corrosion
Understanding how corrosion works helps us prevent it:
1. Barrier Methods:
- Painting: Creates physical barrier between metal and environment
- Oiling/Greasing: Prevents water contact
- Plastic coating: Long-lasting barrier
2. Sacrificial Protection:
- Galvanizing: Coating iron with zinc
- Zinc is more reactive than iron, so it corrodes first
- Sacrificial anodes: Blocks of reactive metal attached to structures
3. Alloying:
- Stainless steel: Chromium forms protective oxide layer
- Bronze: Copper-tin alloy resists corrosion better than pure copper
4. Electroplating:
- Thin layer of unreactive metal (like chromium) electroplated onto reactive metal
- Combines protection with attractive appearance
Why Some Metals Don’t Seem to Corrode
Aluminum’s Trick:
Aluminum actually reacts very rapidly with oxygen, but it forms a thin, invisible layer of aluminum oxide that protects the metal underneath. This is called passivation.
Stainless Steel’s Secret:
The chromium in stainless steel does the same thing – it forms a protective chromium oxide layer.
Gold’s Advantage:
Gold is so unreactive that it simply doesn’t corrode under normal conditions.
Key Chemical Equations You Must Know
Reactivity Series Reactions
Metal + Water:
- 2Na + 2H₂O → 2NaOH + H₂
- Ca + 2H₂O → Ca(OH)₂ + H₂
- Mg + H₂O(steam) → MgO + H₂
Metal + Acid:
- Zn + H₂SO₄ → ZnSO₄ + H₂
- Mg + 2HCl → MgCl₂ + H₂
- Fe + 2HCl → FeCl₂ + H₂
Displacement Reactions:
- Zn + CuSO₄ → ZnSO₄ + Cu
- Fe + CuSO₄ → FeSO₄ + Cu
- Mg + ZnSO₄ → MgSO₄ + Zn
Metal Extraction Equations
Carbon Reduction:
- 2Fe₂O₃ + 3C → 4Fe + 3CO₂
- ZnO + C → Zn + CO
- PbO + C → Pb + CO
Electrolysis:
- Al₂O₃ → 2Al + 3/2O₂ (simplified)
- At cathode: Al³⁺ + 3e⁻ → Al
- At anode: 2O²⁻ → O₂ + 4e⁻
Corrosion Equations
Rusting:
- 4Fe + 3O₂ + 2xH₂O → 2Fe₂O₃.xH₂O
Formation of Protective Layers:
- 4Al + 3O₂ → 2Al₂O₃
- 4Cr + 3O₂ → 2Cr₂O₃
Quick Revision Notes
Properties of Metals
✓ Good conductors of heat and electricity
✓ Malleable and ductile
✓ High density and melting points (mostly)
✓ Lustrous when polished
✓ Form positive ions (cations)
Reactivity Series (Key Points)
✓ Potassium, Sodium, Lithium = Very reactive
✓ Calcium, Magnesium, Aluminum = Reactive
✓ Zinc, Iron, Tin, Lead = Moderately reactive
✓ Copper, Silver, Gold = Unreactive
✓ More reactive metals displace less reactive ones
Extraction Methods
✓ Electrolysis: For metals above carbon (Al, Mg, Na, etc.)
✓ Carbon reduction: For metals below carbon (Fe, Zn, Pb, etc.)
✓ Found native: Very unreactive metals (Au, Ag)
Alloys
✓ Mixtures of metals or metals with non-metals
✓ Usually harder and stronger than pure metals
✓ Examples: Steel (Fe+C), Bronze (Cu+Sn), Brass (Cu+Zn)
Corrosion Prevention
✓ Barrier methods: Paint, oil, plastic coating
✓ Sacrificial protection: Galvanizing, sacrificial anodes
✓ Alloying: Stainless steel
✓ Electroplating: Chromium plating
Common Exam Questions and How to Tackle Them
Question 1: Reactivity Series Order
“Arrange the following metals in order of decreasing reactivity: copper, iron, magnesium, zinc”
Answer Strategy:
- Remember the reactivity series
- Write: Magnesium > Zinc > Iron > Copper
- Always explain: “Based on their position in the reactivity series”
Question 2: Displacement Reactions
“Predict what happens when zinc is added to copper sulfate solution”
Answer Strategy:
- Check reactivity: Zn more reactive than Cu
- Write equation: Zn + CuSO₄ → ZnSO₄ + Cu
- Describe observation: “Blue solution becomes colorless, brown/orange solid forms”
Question 3: Extraction Methods
“Explain why aluminum is extracted by electrolysis but iron by carbon reduction”
Answer Strategy:
- Reference reactivity series
- “Aluminum is above carbon, so carbon cannot displace it”
- “Iron is below carbon, so carbon can displace it”
- Mention energy/cost considerations
Question 4: Corrosion
“State the conditions needed for iron to rust and explain how galvanizing prevents rusting”
Answer Strategy:
- Conditions: Iron + oxygen + water
- Galvanizing: “Coating iron with zinc”
- Zinc more reactive, so corrodes preferentially
- “Sacrificial protection”
Question 5: Alloys
“Explain why alloys are often more useful than pure metals”
Answer Strategy:
- Different sized atoms disrupt regular structure
- Harder for layers to slide over each other
- Results in increased hardness and strength
- Give specific example (e.g., steel vs. pure iron)
Test Yourself: Practice Questions
- A student has four metals: aluminum, copper, iron, and zinc. Arrange these in order of decreasing reactivity and justify your answer.
- Explain why sodium is stored under oil while copper can be left in air.
- A factory wants to extract zinc from zinc oxide. Suggest a suitable method and write the chemical equation.
- Stainless steel contains chromium. Explain how this prevents corrosion.
- Predict what happens when magnesium ribbon is added to copper sulfate solution. Include a balanced chemical equation.
- Explain why electrolysis is expensive compared to carbon reduction for metal extraction.
- A iron ship in seawater has blocks of zinc attached to its hull. Explain why.
Common Mistakes to Avoid
Mistake 1: Confusing Physical and Chemical Properties
Wrong: “Metals are shiny, so they’re reactive”
Correct: Understand that shininess is a physical property, reactivity is chemical
Mistake 2: Memorizing Without Understanding
Wrong: Just memorizing the reactivity series
Correct: Understanding why metals have different reactivities
Mistake 3: Incorrect Displacement Predictions
Wrong: “Copper displaces zinc from zinc sulfate”
Correct: “Zinc displaces copper from copper sulfate” (more reactive displaces less reactive)
Mistake 4: Confusing Extraction Methods
Wrong: “Iron is extracted by electrolysis”
Correct: “Iron is extracted by carbon reduction because it’s below carbon in the reactivity series”
Mistake 5: Incomplete Rusting Conditions
Wrong: “Iron rusts in oxygen”
Correct: “Iron rusts when oxygen AND water are both present”
Your Next Steps to IGCSE Success
Congratulations! You’ve just completed one of the most practical and important topics in IGCSE Chemistry. The knowledge you’ve gained about metals isn’t just academic – it explains the world around you and forms the foundation for understanding industrial processes, environmental issues, and materials science.
Immediate Action Plan:
- Practice the equations until you can write them from memory
- Create flashcards for the reactivity series and alloy compositions
- Work through past paper questions focusing on displacement reactions and extraction methods
- Connect to real life by identifying metals and alloys in your daily environment
Building on This Knowledge:
- Topic 10 (Chemistry of the Environment) builds on corrosion concepts
- Topic 14 (Organic Chemistry) uses similar reaction principles
- Your understanding of electron transfer will be crucial for advanced chemistry
Exam Confidence Builders:
Remember, examiners often ask about the same core concepts in different ways. Master the fundamentals:
- Why metals have different reactivities
- How to predict displacement reactions
- When to use which extraction method
- How alloys improve on pure metals
- Why and how corrosion occurs
The beauty of chemistry lies in its logical patterns. Once you understand why things happen, predicting outcomes becomes much easier. You’re not just memorizing facts – you’re developing chemical intuition that will serve you well beyond IGCSE.
Keep practicing, stay curious, and remember that every expert was once a beginner. You’ve got this!
Recommended –


1 thought on “IGCSE (Cambridge) Chemistry Topic 9: Metals | Your Complete Study Guide”