Introduction to Chemical Bonding
Chemical bonding is the foundation that determines how atoms combine to form compounds and how these compounds behave in different conditions. In AP Chemistry Unit 2– Molecular and Ionic Compound, we explore the intricate relationship between atomic structure and macroscopic properties of substances.
Understanding chemical bonds isn’t just about memorizing definitions-it’s about predicting properties, explaining behavior, and connecting the microscopic world of atoms to the observable properties we measure in the laboratory.
Why This Unit Matters for AP Chemistry
Unit 2 represents approximately 7-9% of the AP Chemistry exam and serves as a crucial bridge between atomic structure (Unit 1) and the properties of substances (Unit 3). The concepts you master here will be essential for success throughout the entire AP Chemistry course.
Types of Chemical Bonds
The Three Primary Bond Types
Chemical bonds form when atoms interact to achieve more stable electron configurations. There are three main types of chemical bonds:
1. Ionic Bonds
- Formation: Complete transfer of electrons from metal to non-metal
- Participants: Typically metals (low electronegativity) and non-metals (high electronegativity)
- Properties: High melting points, conduct electricity when molten or dissolved, brittle
2. Covalent Bonds
- Formation: Sharing of electron pairs between atoms
- Types:
- Polar covalent: Unequal sharing (electronegativity difference 0.4-1.7)
- Nonpolar covalent: Equal sharing (electronegativity difference < 0.4)
- Properties: Variable melting points, generally don’t conduct electricity
3. Metallic Bonds
- Formation: “Sea of electrons” surrounding metal cations
- Participants: Metal atoms only
- Properties: Conductivity, malleability, ductility, metallic luster
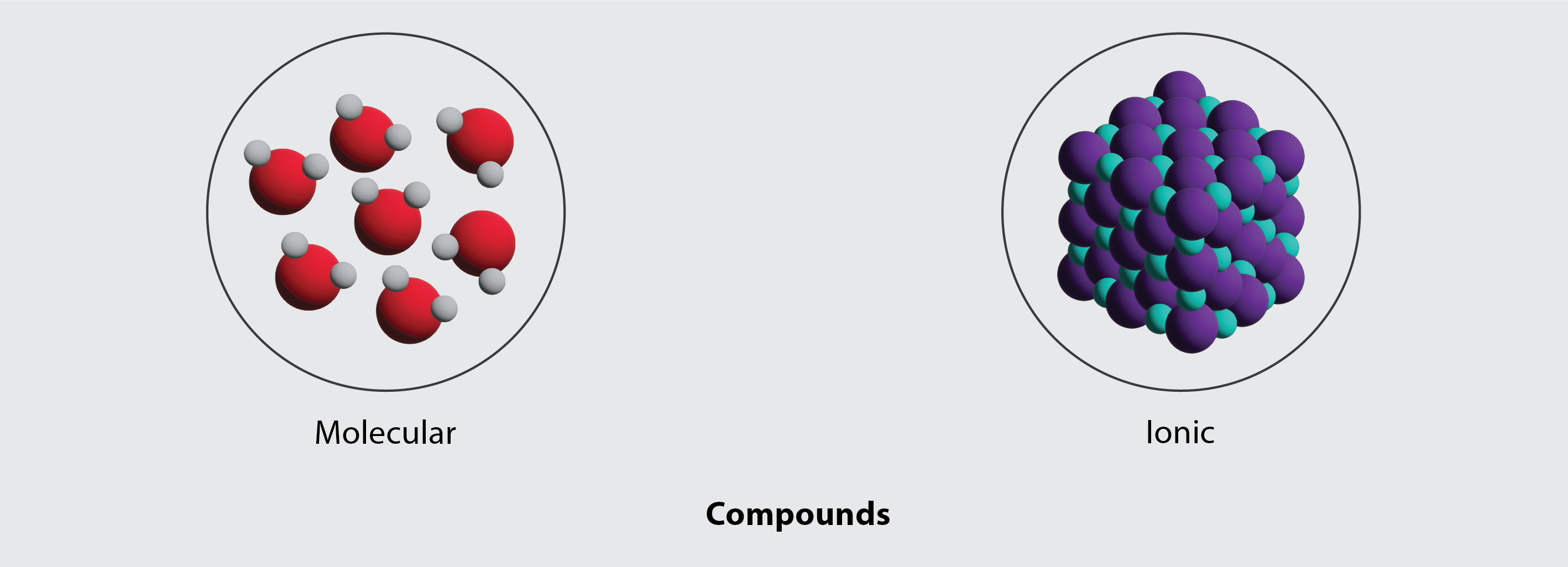
Electronegativity and Bond Polarity
Understanding Electronegativity Trends
Electronegativity is the ability of an atom to attract electrons in a chemical bond. This fundamental property determines bond type and molecular behavior.

Periodic Trends in Electronegativity
Across a Period (Left to Right):
- Electronegativity increases
- Nuclear charge increases, attracting electrons more strongly
- Atomic radius decreases, bringing electrons closer to nucleus
Down a Group (Top to Bottom):
- Electronegativity decreases
- Atomic radius increases, reducing attraction to bonding electrons
- Shielding effect increases
Predicting Bond Type Using Electronegativity
| Electronegativity Difference | Bond Type | Example |
|---|---|---|
| 0.0 – 0.4 | Nonpolar Covalent | H-H (0.0) |
| 0.4 – 1.7 | Polar Covalent | H-Cl (0.9) |
| > 1.7 | Ionic | Na-Cl (2.1) |
Ionic Compounds and Crystal Structures
Formation and Properties
Ionic compounds form when electrons are completely transferred from metals to non-metals, creating charged ions that are held together by electrostatic forces.
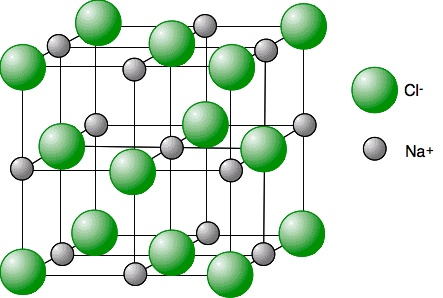
Key Characteristics of Ionic Solids
- 3D Crystal Lattice Structure
- Cations and anions arranged in repeating patterns
- Maximizes attractive forces, minimizes repulsive forces
- Examples: NaCl (cubic), CaF₂ (fluorite structure)
- Coulomb’s Law Application
Force ∝ (q₁ × q₂)/r²- Higher charges = stronger attractions
- Smaller distances = stronger attractions
- Physical Properties
- High melting and boiling points: Strong electrostatic forces
- Brittleness: Shifting layers causes like charges to align
- Electrical conductivity: Only when molten or dissolved
- Solubility: “Like dissolves like” – polar solvents dissolve ionic compounds
Lattice Energy
Lattice energy is the energy required to completely separate one mole of an ionic solid into gaseous ions. It’s directly related to:
- Ion charges: Higher charges = higher lattice energy
- Ion sizes: Smaller ions = higher lattice energy
Molecular Compounds and Lewis Structures
Constructing Lewis Diagrams
Lewis structures are fundamental tools for representing molecular structure and predicting molecular properties.

Step-by-Step Lewis Structure Construction
- Count total valence electrons
- Determine central atom (usually least electronegative, not hydrogen)
- Connect atoms with single bonds
- Complete octets (duet for hydrogen)
- Form multiple bonds if necessary
- Check formal charges
Formal Charge Calculation
Formal Charge = (Valence electrons) - (Lone pair electrons) - (1/2 × Bonding electrons)Rules for using formal charges:
- Sum of formal charges = overall charge
- Best structures have formal charges closest to zero
- Negative formal charges on most electronegative atoms
Resonance Structures
Some molecules cannot be adequately described by a single Lewis structure. Resonance occurs when:
- Multiple valid Lewis structures exist
- Only electron positions differ (not atom positions)
- Actual structure is a hybrid of all resonance forms
Example: Nitrate ion (NO₃⁻) has three equivalent resonance structures.
VSEPR Theory and Molecular Geometry
Valence Shell Electron Pair Repulsion Theory
VSEPR theory predicts molecular geometry based on the idea that electron pairs around a central atom repel each other and adopt arrangements that minimize repulsion.

Common Molecular Geometries
| Electron Pairs | Bonding Pairs | Lone Pairs | Geometry | Bond Angle | Example |
|---|---|---|---|---|---|
| 2 | 2 | 0 | Linear | 180° | CO₂ |
| 3 | 3 | 0 | Trigonal Planar | 120° | BF₃ |
| 3 | 2 | 1 | Bent | ~117° | SO₂ |
| 4 | 4 | 0 | Tetrahedral | 109.5° | CH₄ |
| 4 | 3 | 1 | Trigonal Pyramidal | ~107° | NH₃ |
| 4 | 2 | 2 | Bent | ~104.5° | H₂O |
Molecular Polarity
A molecule is polar if:
- It contains polar bonds (electronegativity differences)
- The molecular geometry is asymmetric (dipoles don’t cancel)
Examples:
- CO₂: Polar bonds, linear geometry → Nonpolar molecule (dipoles cancel)
- H₂O: Polar bonds, bent geometry → Polar molecule (dipoles don’t cancel)
Hybridization
Hybridization explains bond angles and molecular geometry:
- sp³ hybridization: 4 electron pairs, 109.5° angles (tetrahedral)
- sp² hybridization: 3 electron pairs, 120° angles (trigonal planar)
- sp hybridization: 2 electron pairs, 180° angles (linear)
Metallic Bonding and Alloys
The “Sea of Electrons” Model
In metallic bonding:
- Metal atoms release valence electrons
- Electrons form a “sea” that moves freely throughout the structure
- Metal cations are held together by this electron sea
Properties Explained by Metallic Bonding
- Electrical Conductivity: Mobile electrons carry current
- Thermal Conductivity: Electrons transfer kinetic energy
- Malleability and Ductility: Layers can slide without breaking bonds
- Metallic Luster: Free electrons interact with light
Types of Alloys
Substitutional Alloys:
- Foreign atoms replace host atoms in crystal lattice
- Similar-sized atoms (within ~15% size difference)
- Example: Bronze (Cu-Sn)
Interstitial Alloys:
- Small atoms fit into spaces between host atoms
- Significantly different atom sizes
- Example: Steel (Fe-C)
Practice Problems and Applications
Problem 1: Bond Type Prediction
Question: Predict the bond type between the following pairs:
a) Li and F
b) C and H
c) N and N
Solution:
a) Li (0.98) and F (3.98): Difference = 3.0 → Ionic
b) C (2.55) and H (2.20): Difference = 0.35 → Nonpolar covalent
c) N and N: Difference = 0.0 → Nonpolar covalent
Problem 2: Lewis Structure and Geometry
Question: Draw the Lewis structure for SO₂ and predict its molecular geometry.
Solution:
- Total valence electrons: S(6) + 2×O(6) = 18 electrons
- Central atom: S (less electronegative than O)
- Structure: O=S=O with one lone pair on S
- Electron geometry: Trigonal planar
- Molecular geometry: Bent (~119° bond angle)
Problem 3: Lattice Energy Comparison
Question: Which has higher lattice energy: NaCl or MgO?
Solution:
Using Coulomb’s Law:
- NaCl: Na⁺ (+1) and Cl⁻ (-1)
- MgO: Mg²⁺ (+2) and O²⁻ (-2)
MgO has higher charges (2+ and 2- vs 1+ and 1-), so MgO has higher lattice energy.
Exam Tips and Summary
Key Formulas to Remember
- Formal Charge: FC = V – N – B/2
- Coulomb’s Law: F ∝ q₁q₂/r²
- Bond Order: (Bonding electrons – Antibonding electrons)/2
Common Exam Mistakes to Avoid
- Forgetting lone pairs in VSEPR calculations
- Confusing electron geometry with molecular geometry
- Not considering resonance when drawing Lewis structures
- Misapplying electronegativity trends
- Ignoring formal charge when selecting best Lewis structure
Study Strategies
- Practice drawing Lewis structures daily
- Use molecular model kits for 3D visualization
- Create concept maps connecting bonding types to properties
- Work through past AP problems focusing on FRQs
- Form study groups to discuss challenging concepts
Unit 2 Learning Objectives Checklist
✅ 2.1: Explain relationship between bonding type and element properties
✅ 2.2: Represent potential energy vs. distance relationships
✅ 2.3: Model ionic solids using particulate representations
✅ 2.4: Represent metallic solids and alloys
✅ 2.5: Construct accurate Lewis diagrams
✅ 2.6: Apply resonance and formal charge concepts
✅ 2.7: Use VSEPR theory to predict molecular properties
Conclusion
Mastering AP Chemistry Unit 2 requires understanding the fundamental principles that govern how atoms bond and how these bonds determine macroscopic properties. From predicting bond types using electronegativity to visualizing molecular geometry with VSEPR theory, each concept builds upon the previous one.
Remember that chemistry is ultimately about patterns and relationships. The electronegativity trends that help predict bond polarity are the same trends that explain why certain elements form specific types of compounds. The electron arrangements that determine molecular geometry also influence how molecules interact with each other.
As you prepare for the AP exam, focus on understanding these connections rather than memorizing isolated facts. Practice regularly with both multiple-choice and free-response questions, and don’t hesitate to seek help when concepts seem unclear.
Success in Unit 2 sets the foundation for excellence throughout AP Chemistry. Master these concepts, and you’ll be well-prepared for the challenges ahead!
References and Further Reading
- College Board AP Chemistry Course Description
- Khan Academy AP Chemistry Unit 2
- ChemTalk Electronegativity Guide
This comprehensive guide covers all essential concepts in AP Chemistry Unit 2. For personalized help and additional practice problems, consider working with a qualified AP Chemistry tutor or joining a study group. Remember, consistent practice and active engagement with the material are key to success on the AP exam.
Also Read –


2 thoughts on “AP Chemistry Unit 2: Molecular and Ionic Compound Properties-2025”