Stoichiometry: The Recipe Book of Chemistry
Imagine you’re baking a cake. You need exactly 2 cups of flour, 3 eggs, and 1 cup of sugar to make one perfect cake. What happens if you want to make 5 cakes? You’d need 10 cups of flour, 15 eggs, and 5 cups of sugar, right? This is exactly what stoichiometry does in chemistry – it tells us the exact “recipe” for chemical reactions and helps us calculate how much of each substance we need or will produce.
Welcome to Topic 3 of your IGCSE Chemistry journey! Stoichiometry might sound intimidating, but it’s actually one of the most practical and logical topics you’ll encounter. By the end of this guide, you’ll be confidently solving problems that once seemed impossible, and you’ll understand why stoichiometry is considered the backbone of chemistry.
What Exactly Is Stoichiometry?
Stoichiometry comes from two Greek words: “stoicheion” (element) and “metron” (measure). It’s literally the measurement of elements in chemical reactions. Think of it as chemistry’s accounting system – it ensures that what goes into a reaction equals what comes out, following the fundamental law of conservation of mass.
In simple terms, stoichiometry helps us answer questions like:
- How much product can I make from my starting materials?
- How much of each reactant do I need for a complete reaction?
- What’s left over when the reaction is finished?
The Foundation: Understanding the Mole
Before diving into stoichiometric calculations, we need to master the concept of the mole. Don’t worry – it’s not as scary as it sounds!
What Is a Mole?
A mole is simply a counting unit, like a dozen. Just as a dozen always means 12 (whether it’s 12 eggs or 12 cars), a mole always means 6.02 × 10²³ particles (Avogadro’s number). This might seem like a ridiculously large number, but remember – atoms and molecules are incredibly tiny!
Key Mole Relationships:
For any substance:
- 1 mole = 6.02 × 10²³ particles
- 1 mole = relative atomic/molecular mass in grams
- 1 mole of any gas = 24 dm³ at room temperature and pressure (RTP)
The Magic Triangle:
Mass (g)
/ \
/ \
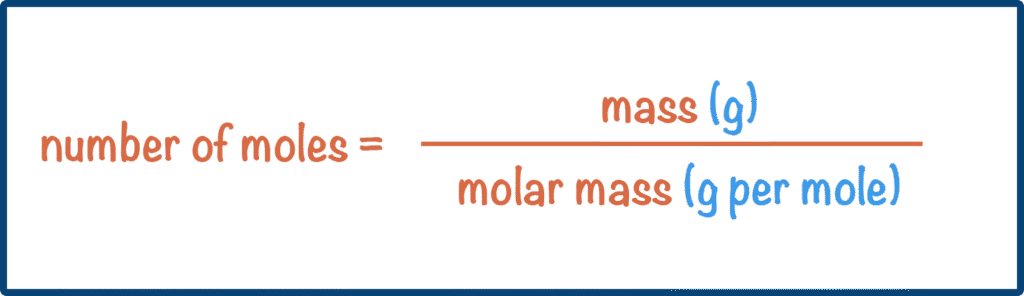
Moles ——————— Mr/ArKey Formulas:
- Moles = Mass ÷ Mr (or Ar)
- Mass = Moles × Mr (or Ar)
- Mr (or Ar) = Mass ÷ Moles
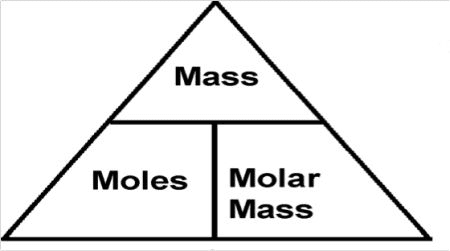
Memory Tip: “Moles Make Mass” – remember the triangle and you’ll never forget these relationships!
Mastering Chemical Equations
Chemical equations are like mathematical equations – they must be balanced. The number of atoms of each element must be the same on both sides.
Step-by-Step Balancing Method:
Example: Balance the equation for the reaction between hydrogen and oxygen to form water.
Step 1: Write the unbalanced equation
H₂ + O₂ → H₂O
Step 2: Count atoms on each side
- Left: 2 H, 2 O
- Right: 2 H, 1 O
Step 3: Balance the most complex molecule first
H₂ + O₂ → 2H₂O
Step 4: Recount and adjust
- Left: 2 H, 2 O
- Right: 4 H, 2 O
Step 5: Balance remaining elements
2H₂ + O₂ → 2H₂O
Final check: 4 H and 2 O on both sides ✓
Common Balancing Mistakes to Avoid:
- Never change the chemical formulas (subscripts)
- Only change the coefficients (numbers in front)
- Always double-check your final answer
- Start with the most complex molecule
Types of Stoichiometric Calculations
1. Mass-to-Mass Calculations
Example Problem: How many grams of water are produced when 4g of hydrogen reacts completely with oxygen?
Given equation: 2H₂ + O₂ → 2H₂O
Solution Steps:
- Find moles of hydrogen: 4g ÷ 2g/mol = 2 moles H₂
- Use mole ratio: From equation, 2 moles H₂ produce 2 moles H₂O
- Calculate mass of water: 2 moles × 18g/mol = 36g
Pro Tip: Always write down the mole ratio from the balanced equation – it’s your roadmap to the answer!
2. Volume-to-Mass Calculations
Example Problem: What mass of carbon dioxide is produced when 48 dm³ of methane burns completely?
Given equation: CH₄ + 2O₂ → CO₂ + 2H₂O
Solution Steps:
- Find moles of methane: 48 dm³ ÷ 24 dm³/mol = 2 moles CH₄
- Use mole ratio: 1 mole CH₄ produces 1 mole CO₂, so 2 moles CH₄ produce 2 moles CO₂
- Calculate mass of CO₂: 2 moles × 44g/mol = 88g
3. Limiting Reagent Problems
Sometimes you’re given amounts of multiple reactants. The limiting reagent is the one that runs out first, determining how much product you can make.
Example Problem: 10g of iron reacts with 5g of sulfur to form iron sulfide. Which is the limiting reagent and how much product forms?
Given equation: Fe + S → FeS
Solution Steps:
- Calculate moles of each reactant:
- Fe: 10g ÷ 56g/mol = 0.179 moles
- S: 5g ÷ 32g/mol = 0.156 moles
- Check the ratio: The equation shows 1:1 ratio, but we have more moles of Fe than S
- Identify limiting reagent: Sulfur (0.156 moles) is limiting
- Calculate product: 0.156 moles FeS × 88g/mol = 13.7g FeS
Percentage Composition and Empirical Formulas
Percentage Composition
This tells us what percentage of a compound’s mass comes from each element.
Formula: % of element = (Mass of element in compound ÷ Total mass of compound) × 100
Example: Find the percentage of carbon in carbon dioxide (CO₂)
- Mass of C in CO₂ = 12g
- Mass of CO₂ = 12 + (2 × 16) = 44g
- % of C = (12 ÷ 44) × 100 = 27.3%
Finding Empirical Formulas
The empirical formula shows the simplest whole number ratio of atoms in a compound.
Step-by-Step Method:
- Convert percentages to grams (assume 100g sample)
- Convert grams to moles
- Divide by the smallest number of moles
- If needed, multiply to get whole numbers
Example: A compound contains 40% carbon, 6.7% hydrogen, and 53.3% oxygen.
Solution:
- Assume 100g sample: 40g C, 6.7g H, 53.3g O
- Convert to moles:
- C: 40 ÷ 12 = 3.33 moles
- H: 6.7 ÷ 1 = 6.7 moles
- O: 53.3 ÷ 16 = 3.33 moles
- Divide by smallest: 3.33 is smallest
- C: 3.33 ÷ 3.33 = 1
- H: 6.7 ÷ 3.33 = 2
- O: 3.33 ÷ 3.33 = 1
- Empirical formula: CH₂O
Key Formulas and Equations Box
Essential Stoichiometry Formulas:
* Moles = Mass ÷ Mr
* Mass = Moles × Mr
* Moles = Volume (dm³) ÷ 24 (at RTP)
* % Composition = (Element mass ÷ Total mass) × 100
* Concentration = Moles ÷ Volume (dm³)
* Number of particles = Moles × 6.02 × 10²³
Remember: Mr = Relative molecular mass
Ar = Relative atomic mass
RTP = Room temperature and pressureCommon Exam Questions and How to Tackle Them
Question Type 1: Basic Mole Calculations
“Calculate the number of moles in 20g of calcium carbonate (CaCO₃)”
Approach:
- Find Mr of CaCO₃: 40 + 12 + (3 × 16) = 100
- Use formula: Moles = 20 ÷ 100 = 0.2 moles
Question Type 2: Mass from Volume
“What mass of hydrogen gas occupies 12 dm³ at RTP?”
Approach:
- Find moles: 12 ÷ 24 = 0.5 moles
- Find mass: 0.5 × 2 = 1g
Question Type 3: Stoichiometric Ratios
“In the reaction N₂ + 3H₂ → 2NH₃, how many grams of ammonia are produced from 14g of nitrogen?”
Approach:
- Find moles of N₂: 14 ÷ 28 = 0.5 moles
- Use ratio: 1 mole N₂ produces 2 moles NH₃
- So 0.5 moles N₂ produces 1 mole NH₃
- Mass of NH₃: 1 × 17 = 17g
Memory Tips and Mnemonics
For Remembering the Mole Triangle:
“My Mother Makes” (Mass = Moles × Mr)
For Balancing Equations:
“Never Change Subscripts, Only Coefficients” – Think of it as “Never Change the DNA of molecules, only how many there are”
For Limiting Reagent Problems:
“The smallest runs out first” – Just like in a race, the slowest (limiting) reagent determines when the reaction stops
Practical Applications: Why Stoichiometry Matters
In Industry:
- Pharmaceutical companies use stoichiometry to ensure exact drug dosages
- Food manufacturers calculate precise ingredient ratios
- Steel production requires exact iron-to-carbon ratios
In Environmental Science:
- Calculating pollution levels and required cleanup amounts
- Determining fuel efficiency and emissions
- Analyzing water treatment chemical requirements
In Everyday Life:
- Cooking (scaling recipes up or down)
- Medicine dosages based on body weight
- Fertilizer application rates for gardens
Quick Revision Notes
Essential Concepts:
- One mole = 6.02 × 10²³ particles = Mr in grams = 24 dm³ of gas at RTP
- Chemical equations must be balanced (same atoms on both sides)
- Mole ratios from balanced equations are crucial for calculations
- Limiting reagent determines maximum product formation
- Empirical formula = simplest whole number ratio of atoms
Calculation Steps:
- Write and balance the chemical equation
- Convert given information to moles
- Use mole ratios from the equation
- Convert back to required units
- Check your answer makes sense
Common Mistakes:
- Forgetting to balance equations first
- Using wrong molar masses
- Mixing up mole ratios
- Not identifying limiting reagents correctly
- Arithmetic errors (always double-check!)
Test Yourself: Practice Questions
Question 1: How many moles are in 32g of oxygen gas (O₂)?
Question 2: Balance this equation: C₄H₁₀ + O₂ → CO₂ + H₂O
Question 3: What volume of carbon dioxide (at RTP) is produced when 6g of carbon burns completely in oxygen?
Question 4: A compound contains 75% carbon and 25% hydrogen by mass. What is its empirical formula?
Answers: 1) 1 mole, 2) 2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O, 3) 12 dm³, 4) CH₄
Your Next Steps to Mastery
Congratulations! You’ve just completed a comprehensive journey through stoichiometry. But learning doesn’t stop here. Here’s how to cement your understanding:
Immediate Actions:
- Work through the practice questions above
- Find 5 past paper questions on stoichiometry and solve them
- Create your own formula sheet with key relationships
This Week:
- Practice one stoichiometry problem daily
- Review your work and identify any recurring mistakes
- Teach a friend or family member about moles – teaching reinforces learning
Ongoing Study:
- Connect stoichiometry to other chemistry topics (energetics, rates, equilibrium)
- Look for real-world examples of stoichiometric principles
- Join online study groups or forums for additional practice
Related Topics to Explore Next
- Topic 4: Electrochemistry – Apply stoichiometry to electron transfer
- Topic 5: Chemical Energetics – Calculate energy changes in reactions
- Topic 8: Acids and Bases – Use mole concepts in neutralization reactions
- Topic 10: Organic Chemistry – Apply empirical formulas to organic compounds
Final Words of Encouragement
Remember, every chemistry expert once struggled with their first stoichiometry problem. The key is persistence and practice. Don’t be discouraged if concepts don’t click immediately – chemistry is a building subject where each topic strengthens your understanding of the next.
Stoichiometry is more than just calculations; it’s the language that helps us understand how the atomic world follows predictable patterns. Master this topic, and you’ll find chemistry becoming more logical and enjoyable.
You’ve got this! With consistent practice and the strategies in this guide, you’ll not only master stoichiometry but also build confidence for your entire IGCSE Chemistry journey. Every balanced equation you write and every mole calculation you complete brings you one step closer to chemistry mastery.
Good luck with your studies, and remember – in chemistry, as in life, balance is everything!
Recommended –

1 thought on “IGCSE (Cambridge) Chemistry Topic 3: Stoichiometry | Your Complete Study Guide”