The Chemical Universe at Your Fingertips
Imagine walking into a massive library where millions of books are scattered randomly across the floor. Finding anything would be nearly impossible, right? Now picture that same library with books perfectly organized by genre, author, and topic. That’s exactly what Dmitri Mendeleev did for chemistry when he created the Periodic Table in 1869!
The Periodic Table isn’t just a chart hanging in your chemistry classroom – it’s the ultimate cheat sheet for understanding how every element in the universe behaves. From the sodium in your table salt to the oxygen you breathe, every element has its designated spot that tells us its story. By the end of this guide, you’ll see the Periodic Table not as a confusing grid of letters and numbers, but as your best friend for IGCSE Chemistry success.
Whether you’re struggling to remember why Group 1 metals are so reactive or wondering how to predict chemical formulas, this comprehensive guide will transform your understanding of Topic 8. Let’s embark on this journey through the most important organizational tool in chemistry!
1: The Foundation – Understanding Atomic Structure
What Makes an Atom Tick?
Before diving into the Periodic Table’s organization, we need to understand what we’re organizing. Every atom consists of three fundamental particles:
Protons: Positively charged particles in the nucleus that determine the element’s identity. The number of protons equals the atomic number – this never changes for a given element.
Neutrons: Neutral particles in the nucleus that affect the atom’s mass but not its chemical properties. Different numbers of neutrons create isotopes.
Electrons: Negatively charged particles orbiting the nucleus in energy levels (shells). These determine how atoms bond and react.
The Magic Numbers: Atomic and Mass Numbers
- Atomic Number (Z): The number of protons in an atom’s nucleus
- Mass Number (A): The total number of protons and neutrons
- Number of Electrons: In a neutral atom, equals the number of protons
Memory Tip: Remember “PAM” – Protons determine the Atomic number, and adding neutrons gives you the Mass number!
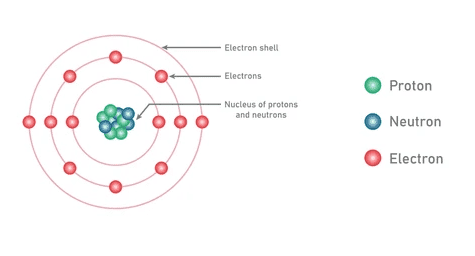
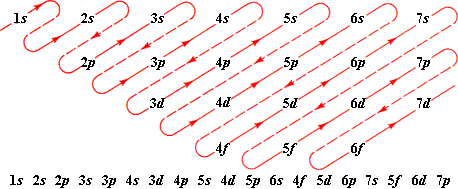
Electron Configuration: The Address System
Electrons don’t randomly float around the nucleus – they occupy specific energy levels (shells) with maximum capacities:
- 1st shell: maximum 2 electrons
- 2nd shell: maximum 8 electrons
- 3rd shell: maximum 8 electrons (for IGCSE level)
Quick Method for Electron Configuration:
- Find the atomic number (number of electrons)
- Fill shells starting from the innermost
- Write as numbers separated by commas
Example: Sodium (Na, atomic number 11) = 2,8,1
2: The Periodic Table’s Brilliant Organization
Mendeleev’s Genius Discovery
Dmitri Mendeleev noticed that when elements were arranged by increasing atomic mass (we now know it’s atomic number), their properties repeated in a predictable pattern. He called this the “Periodic Law” – properties of elements are periodic functions of their atomic numbers.
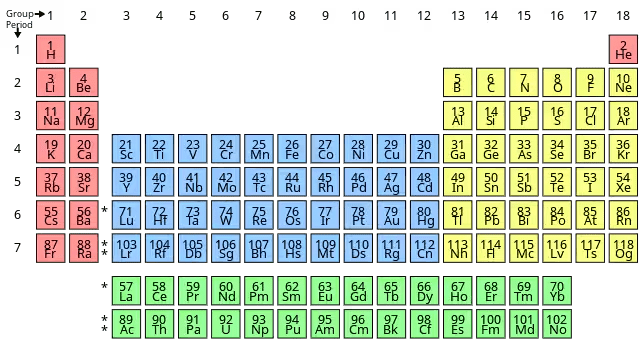
The Modern Periodic Table Structure
Periods (Horizontal Rows):
- Elements in the same period have the same number of electron shells
- Properties change gradually across a period
- There are 7 periods in the complete table
Groups (Vertical Columns):
- Elements in the same group have the same number of outer shell electrons
- Similar chemical properties and reactions
- 18 groups in total (IGCSE focuses on Groups 1, 2, 7, and 8/0)
Key Groups You Must Know
Group 1 – Alkali Metals:
- Lithium (Li), Sodium (Na), Potassium (K)
- One electron in outer shell
- Highly reactive, react vigorously with water
- Form ionic compounds with non-metals
Group 2 – Alkaline Earth Metals:
- Beryllium (Be), Magnesium (Mg), Calcium (Ca)
- Two electrons in outer shell
- Less reactive than Group 1
- Form ionic compounds
Group 7 – Halogens:
- Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I)
- Seven electrons in outer shell
- Very reactive non-metals
- Form ionic compounds with metals, covalent with non-metals
Group 8/0 – Noble Gases:
- Helium (He), Neon (Ne), Argon (Ar)
- Full outer shells (except Helium with 2)
- Extremely unreactive (inert)
- Exist as single atoms
3: Periodic Trends – The Predictable Patterns
Understanding periodic trends is like having a crystal ball for predicting element properties. These patterns repeat predictably across periods and down groups.
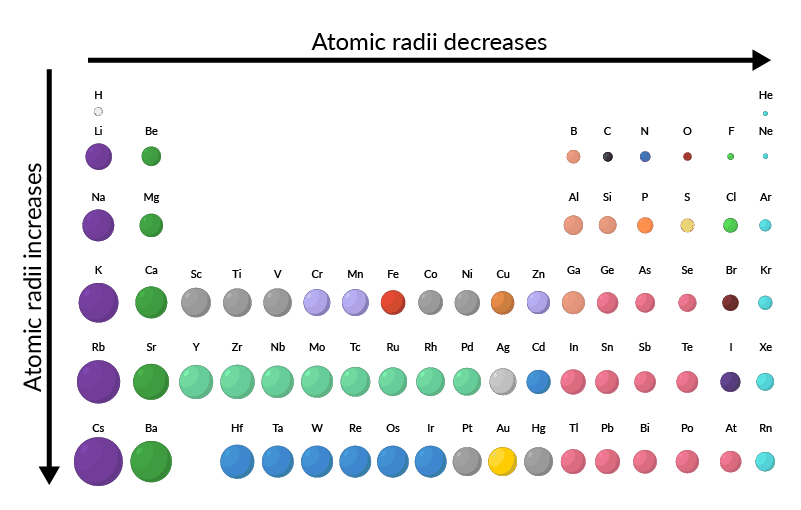
Atomic Radius Trends
Across a Period (Left to Right):
Atomic radius decreases because:
- Nuclear charge increases (more protons)
- Same number of electron shells
- Stronger attraction pulls electrons closer
Down a Group:
Atomic radius increases because:
- Additional electron shells are added
- Outer electrons are further from the nucleus
- Shielding effect reduces nuclear attraction
Ionization Energy Trends
Ionization energy is the energy required to remove an electron from an atom.
Across a Period: Increases (harder to remove electrons as nuclear charge increases)
Down a Group: Decreases (outer electrons are further away and easier to remove)
Electronegativity Trends
Electronegativity measures an atom’s ability to attract electrons in a chemical bond.
Across a Period: Increases (atoms better at attracting electrons)
Down a Group: Decreases (larger atoms have less attraction for bonding electrons)
Memory Trick: “FINe” – Fluorine, which is top-right, has the highest electronegativity!
4: Chemical Properties and Periodic Patterns
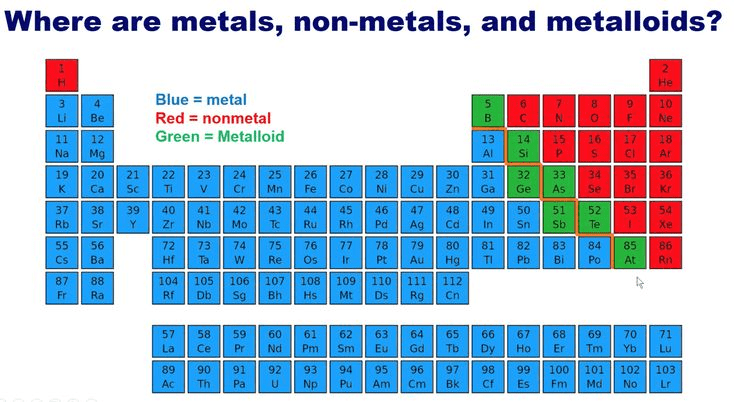
Metallic vs Non-Metallic Character
The Periodic Table shows a clear diagonal divide between metals and non-metals:
Metals (Left side and bottom):
- Lose electrons easily
- Form positive ions (cations)
- Conduct electricity and heat
- Malleable and ductile
Non-metals (Right side and top):
- Gain electrons easily
- Form negative ions (anions)
- Poor conductors (except graphite)
- Brittle when solid
Metalloids: Elements along the diagonal (like Silicon) show properties of both metals and non-metals.
Reactivity Patterns
For Metals: Reactivity increases down a group
- Outer electrons are easier to lose
- Group 1: Li < Na < K (potassium is most reactive)
For Non-metals: Reactivity decreases down a group
- Harder to gain electrons when atoms are larger
- Group 7: F > Cl > Br > I (fluorine is most reactive)
Compound Formation Patterns
The Periodic Table helps predict how elements will combine:
Ionic Compounds: Form between metals and non-metals
- Metal loses electrons → positive ion
- Non-metal gains electrons → negative ion
- Examples: NaCl, MgO, CaF₂
Covalent Compounds: Form between non-metals
- Electrons are shared
- Examples: H₂O, CO₂, NH₃
5: Group Properties in Detail
Group 1 – The Alkali Metals
These metals are the “rebels” of the Periodic Table – they hate being alone and react dramatically with water!
Physical Properties:
- Soft (can be cut with a knife)
- Low melting points (decrease down the group)
- Low density (lithium floats on water!)
- Shiny when freshly cut, tarnish quickly
Chemical Properties:
- All have 1 electron in outer shell
- Form ionic compounds with charge +1
- React vigorously with water: 2Na + 2H₂O → 2NaOH + H₂
- Reactivity increases down the group
Safety Note: Never touch alkali metals with bare hands – they react with moisture in your skin!
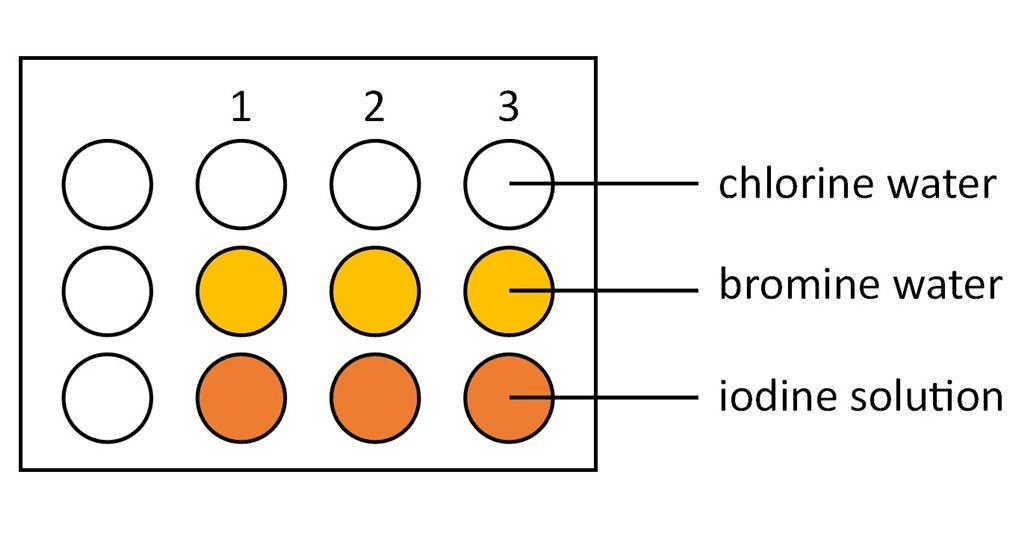
Group 7 – The Halogens
Think of halogens as the “electron hunters” – they desperately want one more electron to complete their outer shell.
Physical Properties at Room Temperature:
- F₂: Pale yellow gas
- Cl₂: Green gas
- Br₂: Red-brown liquid
- I₂: Purple-black solid
Chemical Properties:
- All have 7 electrons in outer shell
- Form ionic compounds with metals (charge -1)
- Form covalent compounds with non-metals
- Exist as diatomic molecules (F₂, Cl₂, etc.)
- Reactivity decreases down the group
Displacement Reactions:
More reactive halogens displace less reactive ones:
Cl₂ + 2KBr → 2KCl + Br₂
Group 8/0 – The Noble Gases
These are the “loners” of the Periodic Table – they don’t need friends because they’re already complete!
Why Are They So Unreactive?
- Full outer electron shells
- Stable electronic configuration
- No tendency to gain or lose electrons
Uses:
- Helium: Balloons, diving mixtures
- Neon: Signs and lights
- Argon: Light bulbs, welding
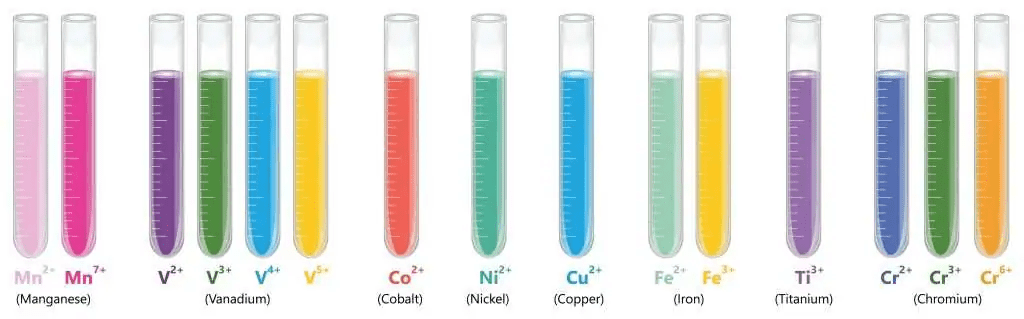
6: Transition Elements
Between Groups 2 and 3, you’ll find the transition elements – the “multitaskers” of the Periodic Table.
Key Properties:
- Multiple oxidation states (can form different charged ions)
- Colored compounds
- Good catalysts
- High density and strength
Examples:
- Iron (Fe): Forms Fe²⁺ and Fe³⁺ ions
- Copper (Cu): Forms Cu⁺ and Cu²⁺ ions
- Used in industry as catalysts
7: Exam Success Strategies
Common Exam Question Types
1. Electron Configuration Questions
“Give the electron configuration of chlorine (Cl, atomic number 17)”
Answer: 2,8,7
2. Trend Explanation Questions
“Explain why atomic radius decreases across Period 2”
Answer: Nuclear charge increases while electron shells remain the same, so electrons are pulled closer to the nucleus.
3. Property Prediction Questions
“Predict the properties of francium (Fr) in Group 1”
Answer: Most reactive alkali metal, lowest melting point, largest atomic radius.
Common Mistakes to Avoid
Mistake 1: Confusing periods with groups
Solution: Remember periods are horizontal (like sitting in rows), groups are vertical (like standing in groups)
Mistake 2: Getting reactivity trends backwards
Solution: Metals get MORE reactive going DOWN, non-metals get LESS reactive going DOWN
Mistake 3: Forgetting diatomic molecules
Solution: Remember “BrINCl HOF” – these seven elements exist as diatomic molecules
Mistake 4: Mixing up atomic number and mass number
Solution: Atomic number = protons only, Mass number = protons + neutrons
Key Formulas and Equations Box
Important Chemical Equations:
- Alkali Metal + Water:
2M + 2H₂O → 2MOH + H₂
(where M = Li, Na, K) - Halogen Displacement:
X₂ + 2MY → 2MX + Y₂
(where X is more reactive than Y) - Ionic Compound Formation:
Metal + Non-metal → Ionic compound
Na + Cl → NaCl - Ion Formation:
- Metal atoms → Positive ions (lose electrons)
- Non-metal atoms → Negative ions (gain electrons)
Key Formulas:
- Mass number (A) = Number of protons + Number of neutrons
- In neutral atoms: Number of protons = Number of electrons
- Atomic number (Z) = Number of protons
Quick Revision Notes
Essential Facts to Remember:
- Periodic Law: Properties repeat periodically when elements are arranged by atomic number
- Group Patterns:
- Same number of outer electrons
- Similar chemical properties
- Predictable trends in physical properties
- Period Patterns:
- Same number of electron shells
- Properties change gradually across
- Reactivity Trends:
- Metals: Increase down groups
- Non-metals: Decrease down groups
- Key Groups:
- Group 1: 1 outer electron, very reactive
- Group 7: 7 outer electrons, very reactive
- Group 8: Full outer shells, unreactive
- Atomic Trends:
- Size decreases across periods, increases down groups
- Ionization energy increases across periods, decreases down groups
Test Yourself – Practice Questions
Quick Check Questions:
- What is the electron configuration of aluminum (Al, atomic number 13)?
- Why does chlorine react more vigorously than iodine?
- Predict the formula of the compound formed between magnesium and fluorine.
- Explain why Group 8 elements are unreactive.
- Which element would you expect to have properties similar to lithium?
Exam-Style Questions:
- Element X has atomic number 19.
a) Give its electron configuration
b) Predict which group it belongs to
c) Describe how it would react with water - Explain the trend in atomic radius across Period 3, referring to nuclear charge and electron shielding.
Answers:
- 2,8,3
- Chlorine has a smaller atomic radius, so it attracts electrons more strongly
- MgF₂
- They have full outer electron shells
- Other Group 1 elements (Na, K, Rb, Cs, Fr)
- a) 2,8,8,1 b) Group 1 c) Reacts vigorously, produces hydrogen gas and hydroxide
- Decreases because nuclear charge increases while electron shells remain the same, pulling electrons closer
8: Real-World Applications
The Periodic Table in Everyday Life
In Your Kitchen:
- Sodium chloride (table salt) – Group 1 + Group 7
- Aluminum foil – Group 3 metal
- Fluoride in toothpaste – Group 7 element
In Technology:
- Silicon in computer chips – Group 4 metalloid
- Lithium in batteries – Group 1 metal
- Noble gases in LED lights – Group 8 elements
In Medicine:
- Iodine as antiseptic – Group 7 element
- Calcium supplements – Group 2 metal
- Radioactive isotopes for medical imaging
Environmental Connections
Understanding the Periodic Table helps explain environmental issues:
- Ozone depletion: Involves Group 7 elements (chlorine, bromine)
- Acid rain: Contains sulfur and nitrogen compounds
- Water treatment: Uses chlorine (Group 7) for disinfection
Advanced Tips for Top Grades
Linking Concepts
Connect electron configuration to bonding:
- Outer electrons determine bonding behavior
- Full shells = stability = unreactivity
- Nearly full/empty shells = high reactivity
Link atomic structure to physical properties:
- More protons = stronger nuclear attraction = smaller atoms
- More electron shells = larger atoms = different properties
Analytical Thinking
When facing unfamiliar elements:
- Find the group and period
- Predict electron configuration
- Compare to known elements in same group
- Apply periodic trends
Memory Techniques
Group 7 Colors: “Fair Children Bring Ice”
- Fluorine: Pale yellow (Fair)
- Chlorine: Green (Children)
- Bromine: Red-brown (Bring)
- Iodine: Purple-black (Ice)
Reactivity Series: Create stories linking reactive elements to dramatic scenarios
Common Misconceptions Clarified
Misconception 1: “Heavier atoms are always larger”
Reality: Atomic radius can decrease across periods despite increasing mass due to increased nuclear charge.
Misconception 2: “All metals are the same”
Reality: Metals show huge variation in reactivity, from highly reactive alkali metals to unreactive noble metals like gold.
Misconception 3: “The Periodic Table is just for memorization”
Reality: It’s a predictive tool that helps you understand and predict chemical behavior.
Your Path to Mastery
Active Learning Strategies
- Create your own periodic table: Draw it from memory, adding electron configurations
- Use mnemonics: Develop memory aids for trends and properties
- Teach others: Explain concepts to classmates or family
- Practice predictions: Given an element, predict all its properties
- Connect to real life: Find periodic table elements in everyday objects
Exam Preparation Tips
Two weeks before exam:
- Review all key trends and exceptions
- Practice drawing electron configurations quickly
- Memorize key group properties
Conclusion: Your Chemical Journey Continues
Congratulations! You’ve just completed a comprehensive journey through IGCSE Chemistry Topic 8. The Periodic Table is no longer a mysterious grid of letters and numbers – it’s your roadmap to understanding the chemical universe.
Remember, the Periodic Table tells a story. Each element has its place for a reason, and those reasons follow predictable patterns. When you understand these patterns, chemistry becomes less about memorization and more about logical thinking.
As you continue your chemistry studies, you’ll discover that Topic 8 connects to everything else. Bonding patterns, reaction predictions, compound formulas – they all stem from an element’s position in the Periodic Table. You now have the foundation to excel not just in this topic, but in chemistry as a whole.
Your Next Steps:
- Practice regularly: Use the test questions provided and seek out more from past papers
- Connect topics: Link periodic trends to bonding, acids and bases, and organic chemistry
- Stay curious: Investigate how new synthetic elements are added to the table
- Build confidence: Remember that understanding patterns is more powerful than memorizing facts
The Periodic Table has guided chemists for over 150 years, and now it’s your turn to use this powerful tool. Whether you’re predicting reaction products, explaining chemical behavior, or solving complex problems, let the Periodic Table be your trusted companion.
Keep practicing, stay curious, and remember – every expert was once a beginner. You’ve got this! Your success in IGCSE Chemistry starts with mastering the beautiful, logical world of the Periodic Table.
Ready for your next challenge? Explore how these periodic trends influence chemical bonding in Topic 5, or discover how they affect the behavior of acids and bases in Topic 7. The chemical world is waiting for you!
Recommended –

1 thought on “IGCSE (Cambridge) Chemistry Topic 8: The Periodic Table | Your Complete Study Guide”