Welcome to AP Chemistry Unit 6: Thermochemistry, arguably one of the most practical and applicable units in your entire AP Chemistry journey. This isn’t just about memorizing formulas or balancing equations; it’s about understanding the fundamental energy principles that govern every chemical process around us, from the metabolism in your cells to the combustion in car engines.
Why Thermochemistry Matters
Picture this: You’re holding a hand warmer on a cold winter day, feeling the gentle heat spread through your fingers. Or maybe you’ve watched a spectacular fireworks display, marveling at the explosive energy released in brilliant colors across the night sky. What you’re witnessing in both scenarios is thermochemistry in action – the fascinating study of energy changes that occur during chemical reactions.
The Challenge You Face
If you’re like most AP Chemistry students, thermochemistry might seem intimidating at first. The combination of mathematical calculations, conceptual understanding, and application to real-world scenarios can feel overwhelming. Many students struggle with:
- Understanding the difference between enthalpy, entropy, and Gibbs free energy
- Mastering calorimetry calculations and experimental design
- Applying Hess’s Law to multi-step problems
- Connecting molecular-level interactions to macroscopic energy changes
- Managing time effectively during thermochemistry problems on the AP exam
Your Path to Success
But here’s the good news: thermochemistry follows logical patterns and principles that, once mastered, will not only help you excel on the AP exam but also provide you with a deeper appreciation for the energy processes that power our world. This comprehensive guide will take you from confusion to confidence, providing you with:
- Crystal-clear explanations of core concepts
- Step-by-step problem-solving strategies
- 25 practice problems with detailed solutions
- Memory techniques and study schedules
- Real-world connections that make learning meaningful
- Expert exam strategies from experienced AP Chemistry teachers
By the end of this guide, you’ll not only understand thermochemistry – you’ll think like a thermochemist, approaching energy problems with confidence and precision.
Quick Reference Guide
Key Formulas at a Glance
| Concept | Formula | Units | When to Use |
|---|---|---|---|
| Heat Transfer | q = mcΔT | Joules (J) | Temperature changes, no phase change |
| Enthalpy Change | ΔH = H(products) – H(reactants) | kJ/mol | Energy change in reactions |
| Calorimetry | q = CΔT | Joules (J) | Using calorimeter data |
| Hess’s Law | ΔH(total) = ΣΔH(steps) | kJ/mol | Multi-step reaction calculations |
| Formation Enthalpy | ΔH°rxn = ΣΔH°f(products) – ΣΔH°f(reactants) | kJ/mol | Standard condition calculations |
Essential Constants & Values
- Specific Heat of Water: 4.18 J/g·°C
- Standard Temperature: 25°C (298 K)
- Standard Pressure: 1 atm (101.3 kPa)
- Avogadro’s Number: 6.022 × 10²³ mol⁻¹
Quick Concept Check
Exothermic vs Endothermic
- Exothermic: ΔH < 0 (negative), releases heat, products lower in energy
- Endothermic: ΔH > 0 (positive), absorbs heat, products higher in energy
State Functions vs Path Functions
- State Functions: Enthalpy (H), depend only on initial and final states
- Path Functions: Heat (q), depend on the specific pathway taken
Foundation Concepts
Understanding Energy in Chemical Systems
Before diving into complex calculations, let’s establish the fundamental principles that govern all thermochemical processes. Think of thermochemistry as the accounting department of chemistry – it keeps track of energy “deposits” and “withdrawals” during chemical reactions.
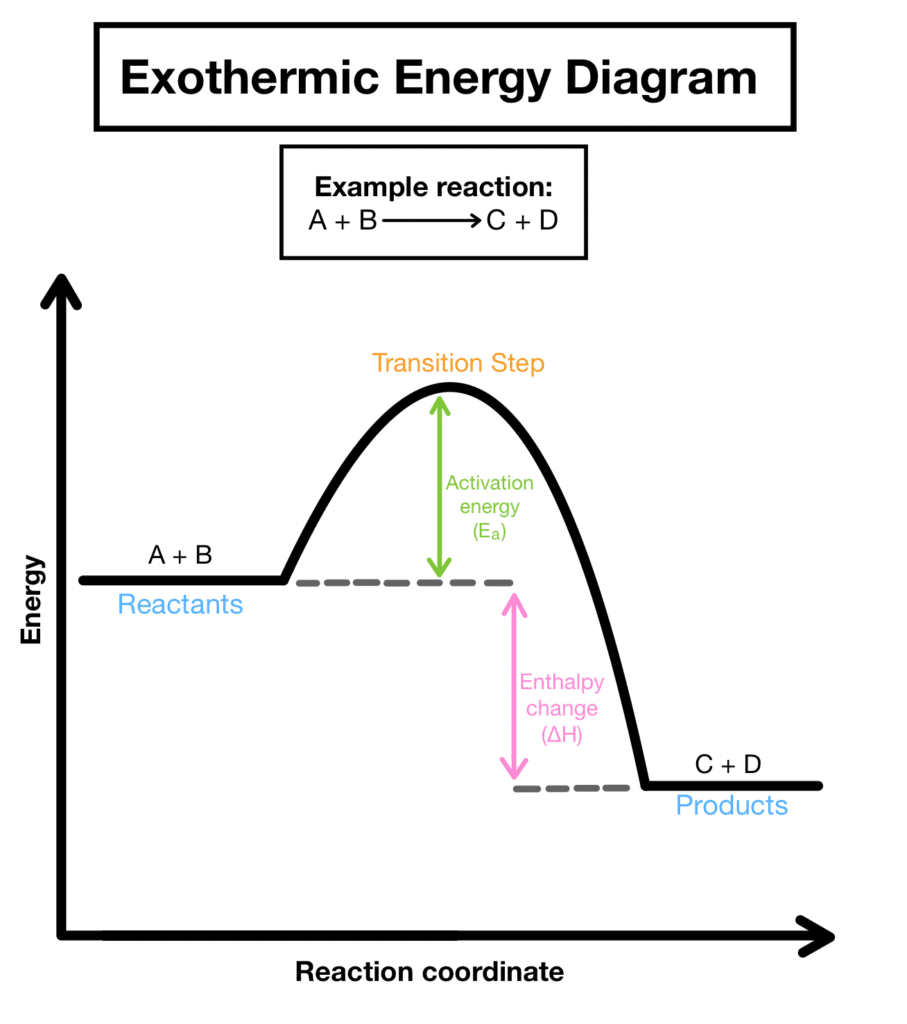
The First Law of Thermodynamics
The foundation of all thermochemical thinking rests on the First Law of Thermodynamics: Energy cannot be created or destroyed, only transferred or converted from one form to another. This principle, also known as the law of conservation of energy, means that in any chemical process, we must account for every joule of energy.
When you light a match, the chemical potential energy stored in the match head doesn’t disappear – it transforms into heat, light, and the kinetic energy of product molecules. The total energy before and after remains constant, but its distribution changes dramatically.
System vs. Surroundings: Drawing the Line
In thermochemistry, we must clearly define our system (the part of the universe we’re studying) and the surroundings (everything else). This distinction is crucial because it determines the sign and magnitude of energy changes we measure.
System: Usually the chemical reaction or the contents of our reaction vessel
Surroundings: The laboratory, the calorimeter, the air around us
Energy flows between system and surroundings, and our measurements depend on our perspective. When a reaction releases heat (exothermic), the system loses energy while the surroundings gain it.
Heat vs. Temperature: The Critical Distinction
Many students confuse heat and temperature, but understanding their difference is crucial for thermochemistry success:
Temperature: A measure of average kinetic energy of particles in a substance
Heat: The transfer of energy between objects at different temperatures
Think of temperature as the “intensity” of thermal energy, while heat is the “quantity” being transferred. A small match flame has a high temperature but contains little heat, while a warm swimming pool has a lower temperature but contains enormous amounts of thermal energy.
Detailed Topic Breakdown
1. Enthalpy (ΔH): The Heart of Thermochemistry
Enthalpy represents the total heat content of a system at constant pressure – the conditions under which most chemical reactions occur in open containers. Unlike internal energy, enthalpy accounts for both the internal energy of the system and the work done by the system against external pressure.
Standard Enthalpy Changes
Standard Enthalpy of Formation (ΔH°f)
The enthalpy change when one mole of a compound is formed from its constituent elements in their standard states. By definition, ΔH°f for elements in their standard states equals zero.
Examples:
- H₂(g) + ½O₂(g) → H₂O(l) ΔH°f = -285.8 kJ/mol
- C(graphite) + O₂(g) → CO₂(g) ΔH°f = -393.5 kJ/mol
Standard Enthalpy of Combustion (ΔH°c)
The enthalpy change when one mole of a substance undergoes complete combustion in oxygen under standard conditions.
Standard Enthalpy of Fusion and Vaporization
- ΔH°fus: Energy required to melt one mole of solid
- ΔH°vap: Energy required to vaporize one mole of liquid
Bond Enthalpy and Molecular Thinking
Understanding reactions at the molecular level helps explain why certain reactions are exothermic while others are endothermic. Every chemical reaction involves breaking existing bonds (endothermic process) and forming new bonds (exothermic process).
Net Energy Change = Energy Required to Break Bonds – Energy Released in Forming Bonds
When more energy is released in bond formation than required for bond breaking, the reaction is exothermic. The opposite produces an endothermic reaction.
2. Calorimetry: Measuring Heat in the Laboratory
Calorimetry provides the experimental foundation for thermochemistry. By carefully measuring temperature changes in controlled systems, we can calculate the energy changes accompanying chemical reactions.
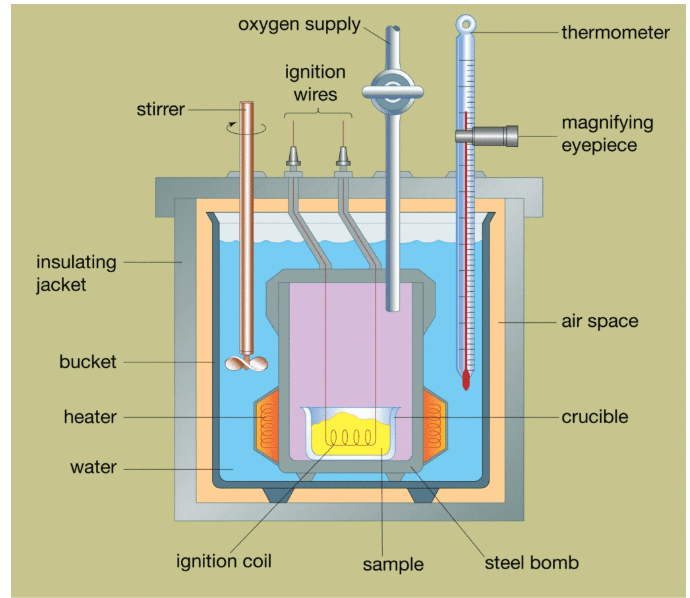
Coffee Cup Calorimetry
The simplest calorimeter consists of nested styrofoam cups, a thermometer, and a stirrer. This setup approximates a constant-pressure calorimeter, making it ideal for measuring enthalpy changes in solution reactions.
Key Assumptions in Coffee Cup Calorimetry:
- No heat is lost to the surroundings (perfect insulation)
- The calorimeter itself absorbs negligible heat
- The solution has the same specific heat as pure water
- The pressure remains constant throughout the experiment
Calculation Strategy:
- Calculate heat absorbed by the solution: q = mcΔT
- Account for the calorimeter’s heat capacity if significant
- Apply conservation of energy: qrxn = -qsolution
Bomb Calorimetry
For combustion reactions, bomb calorimetry provides more accurate measurements. The reaction occurs in a sealed, rigid container (the “bomb”) submerged in a known mass of water.
Key Differences from Coffee Cup Calorimetry:
- Constant volume instead of constant pressure
- Measures internal energy change (ΔE) rather than enthalpy change (ΔH)
- Requires correction for the difference: ΔH = ΔE + ΔnRT
3. Hess’s Law: The Thermochemical GPS
Hess’s Law states that the total enthalpy change for a reaction is independent of the pathway taken. This principle allows us to calculate enthalpy changes for reactions that are difficult or impossible to measure directly.
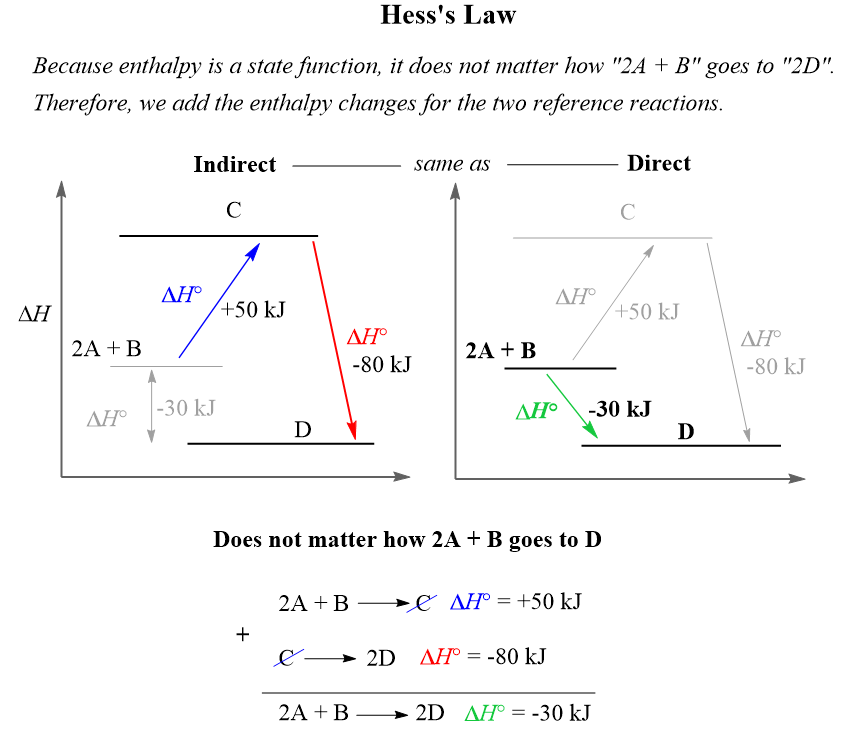
Hess’s Law demonstrates that enthalpy is a state function
The Mathematical Foundation
Because enthalpy is a state function, we can write:
ΔH(direct) = ΔH(step 1) + ΔH(step 2) + ΔH(step 3) + …
This relationship holds regardless of the number of intermediate steps or the complexity of the pathway.
Strategic Approaches to Hess’s Law Problems
Method 1: Algebraic Manipulation
Treat thermochemical equations like algebraic equations:
- Reverse a reaction → Change the sign of ΔH
- Multiply a reaction by a factor → Multiply ΔH by the same factor
- Add reactions → Add their ΔH values
Method 2: Formation Enthalpies
Use standard formation enthalpies to calculate reaction enthalpies:
ΔH°rxn = Σ(nΔH°f)products – Σ(nΔH°f)reactants
Method 3: Energy Cycles
Create diagrams showing different pathways between reactants and products, then apply the principle that all pathways must give the same total energy change.
4. Thermodynamic Spontaneity: Beyond Enthalpy
While Unit 6 focuses primarily on enthalpy, understanding the broader context of thermodynamic spontaneity provides crucial insight into why reactions occur.
Entropy (ΔS): The Disorder Factor
Entropy measures the dispersal of energy and matter in a system. The Second Law of Thermodynamics states that the total entropy of the universe always increases for spontaneous processes.
Factors Increasing Entropy:
- Phase changes: solid → liquid → gas
- Increase in number of particles
- Temperature increase
- Mixing of substances
Gibbs Free Energy (ΔG): The Ultimate Predictor
The Gibbs free energy change determines reaction spontaneity:
ΔG = ΔH – TΔS
- ΔG < 0: Spontaneous reaction
- ΔG > 0: Non-spontaneous reaction
- ΔG = 0: Equilibrium condition
Problem-Solving Mastery
Strategic Problem-Solving Framework
Successful thermochemistry problem-solving follows a systematic approach. Here’s the framework used by top AP Chemistry students:
The IDEAL Method
Identify what type of problem you’re dealing with
Determine what information is given and what you need to find
Establish the relevant equations and principles
Apply the mathematics carefully with proper units
Look back and check if your answer makes sense
Multiple Choice Practice Questions
Question 1: Which of the following statements about enthalpy is correct?
A) Enthalpy is always positive for combustion reactions
B) Enthalpy change depends on the pathway taken
C) Enthalpy is a state function
D) Enthalpy and heat are identical concepts
Answer: C – Enthalpy is a state function, meaning it depends only on the initial and final states, not the pathway taken.
Question 2: For the reaction 2H₂(g) + O₂(g) → 2H₂O(l), ΔH = -572 kJ. What is the enthalpy change for H₂O(l) → H₂(g) + ½O₂(g)?
A) -572 kJ
B) -286 kJ
C) +286 kJ
D) +572 kJ
Answer: C – Reversing the reaction changes the sign, and we need the enthalpy per mole of water (divide by 2).
Question 3: In a coffee cup calorimeter, 50.0 mL of 1.0 M HCl is mixed with 50.0 mL of 1.0 M NaOH. The temperature increases from 22.0°C to 28.5°C. What is the enthalpy of neutralization? (Assume density = 1.0 g/mL and specific heat = 4.18 J/g·°C)
A) -54.2 kJ/mol
B) -27.1 kJ/mol
C) +27.1 kJ/mol
D) +54.2 kJ/mol
Answer: A – q = (100.0 g)(4.18 J/g·°C)(6.5°C) = 2717 J = 2.72 kJ. For 0.050 mol reaction: ΔH = -2.72 kJ/0.050 mol = -54.4 kJ/mol
Question 4: Given the following reactions:
C(s) + O₂(g) → CO₂(g) ΔH = -394 kJ
2CO(g) + O₂(g) → 2CO₂(g) ΔH = -566 kJ
What is ΔH for C(s) + ½O₂(g) → CO(g)?
A) -111 kJ
B) -222 kJ
C) +111 kJ
D) +222 kJ
Answer: A – Using Hess’s Law: ΔH = -394 – (-566/2) = -394 + 283 = -111 kJ
Question 5: Which process has the largest positive ΔS?
A) H₂O(s) → H₂O(l)
B) H₂O(l) → H₂O(g)
C) 2H₂(g) + O₂(g) → 2H₂O(l)
D) NaCl(s) → Na⁺(aq) + Cl⁻(aq)
Answer: B – Vaporization involves the largest increase in molecular disorder.
Free Response Problems with Step-by-Step Solutions
Problem 1: Calorimetry and Enthalpy Calculation
A student performs an experiment to determine the enthalpy of combustion of ethanol. In a bomb calorimeter with a calorimeter constant of 2.45 kJ/°C, 0.500 g of ethanol (C₂H₅OH) is completely combusted, causing the temperature to rise from 20.00°C to 23.65°C.
(a) Calculate the heat released in this combustion.
Solution:
Heat released = Calorimeter constant × ΔT
Heat released = 2.45 kJ/°C × (23.65 – 20.00)°C = 2.45 × 3.65 = 8.94 kJ
(b) Calculate the molar enthalpy of combustion of ethanol.
Solution:
First, find moles of ethanol:
Molar mass of C₂H₅OH = 2(12.01) + 6(1.008) + 16.00 = 46.07 g/mol
Moles = 0.500 g ÷ 46.07 g/mol = 0.0109 mol
Molar enthalpy of combustion = -8.94 kJ ÷ 0.0109 mol = -820 kJ/mol
(Negative because combustion is exothermic)
(c) Write the balanced thermochemical equation for this combustion.
Solution:
C₂H₅OH(l) + 3O₂(g) → 2CO₂(g) + 3H₂O(l) ΔH = -820 kJ/mol
Problem 2: Hess’s Law Application
Given the following thermochemical equations:
- N₂(g) + 3H₂(g) → 2NH₃(g) ΔH₁ = -92.4 kJ
- N₂(g) + 2H₂(g) → N₂H₄(l) ΔH₂ = +50.6 kJ
- H₂(g) + ½O₂(g) → H₂O(l) ΔH₃ = -285.8 kJ
Calculate ΔH for: N₂H₄(l) + O₂(g) → N₂(g) + 2H₂O(l)
Solution:
Target equation: N₂H₄(l) + O₂(g) → N₂(g) + 2H₂O(l)
We need to manipulate the given equations:
- Reverse equation 2: N₂H₄(l) → N₂(g) + 2H₂(g) ΔH = -50.6 kJ
- Use equation 3 twice: 2H₂(g) + O₂(g) → 2H₂O(l) ΔH = 2(-285.8) = -571.6 kJ
Adding these: N₂H₄(l) + O₂(g) → N₂(g) + 2H₂O(l)
ΔH = -50.6 + (-571.6) = -622.2 kJ
Study Strategies & Exam Preparation
Memory Techniques for Thermochemistry
The “Energy Story” Method
Create narratives for each type of reaction:
- Exothermic reactions: “Energy escapes the system” (ΔH negative)
- Endothermic reactions: “Energy enters the system” (ΔH positive)
Mnemonic Devices
For State Functions: “Henry Can’t See Giraffes”
(Enthalpy, Entropy, Temperature, Gibbs free energy are state functions)
For Calorimetry: “My Cat Drinks Tea”
(Mass × Specific heat × ΔTemperature = heat)
Recommended Study Schedule
4-Week Preparation Plan
Week 1: Foundation Building
- Day 1-2: Energy concepts and terminology
- Day 3-4: Enthalpy and thermochemical equations
- Day 5-6: Basic calorimetry calculations
- Day 7: Review and practice problems
Week 2: Core Concepts
- Day 1-2: Coffee cup calorimetry
- Day 3-4: Bomb calorimetry
- Day 5-6: Introduction to Hess’s Law
- Day 7: Mixed practice problems
Week 3: Advanced Applications
- Day 1-2: Complex Hess’s Law problems
- Day 3-4: Formation and combustion enthalpies
- Day 5-6: Thermodynamic spontaneity
- Day 7: Comprehensive review
Week 4: Exam Preparation
- Day 1-2: Timed practice tests
- Day 3-4: Review weak areas
- Day 5-6: Final review and confidence building
- Day 7: Rest and exam readiness
Common Mistakes and How to Avoid Them
Sign Errors in Thermochemistry
Common Mistake: Forgetting that exothermic reactions have negative ΔH values
Solution: Always think about energy flow – does energy leave (negative) or enter (positive) the system?
Unit Conversion Errors
Common Mistake: Mixing J and kJ in calculations
Solution: Always convert to the same units before calculating, and double-check your final answer units
Calorimetry Assumption Errors
Common Mistake: Using the wrong mass in q = mcΔT calculations
Solution: Remember to use the total mass of the solution, not just the mass of the limiting reagent
Time Management Strategies for the AP Exam
For Multiple Choice Questions (90 minutes, 60 questions)
- Spend no more than 1.5 minutes per question
- Flag difficult questions and return to them
- Use process of elimination aggressively
- Look for answer choices that differ by simple factors (2, ½, etc.)
For Free Response Questions (105 minutes, 7 questions)
- Read all questions first and tackle easiest ones first
- Show all work clearly – partial credit is available
- Use proper significant figures and units
- Leave time for a final review
Advanced Applications & Real-World Connections
Thermochemistry in Everyday Life
Food and Metabolism
Every calorie listed on food packaging represents the energy content measured through bomb calorimetry. When you metabolize glucose, your body performs the same thermochemical reaction that occurs in a bomb calorimeter, just at body temperature with enzymes as catalysts:
C₆H₁₂O₆(s) + 6O₂(g) → 6CO₂(g) + 6H₂O(l) ΔH = -2802 kJ/mol
This energy powers everything from muscle contractions to brain function, demonstrating thermochemistry’s fundamental role in life itself.
Environmental Applications
Global Warming and Thermochemistry
The greenhouse effect is fundamentally a thermochemical phenomenon. CO₂ and other greenhouse gases absorb infrared radiation (heat) and re-emit it, trapping energy in Earth’s atmosphere. Understanding enthalpy changes in combustion reactions helps us calculate the environmental impact of burning fossil fuels.
Alternative Energy Sources
- Hydrogen fuel cells: 2H₂(g) + O₂(g) → 2H₂O(l) ΔH = -572 kJ
- Solar panel efficiency: Converting light energy to electrical energy
- Battery technology: Storing and releasing chemical potential energy
Industrial Applications
Chemical Manufacturing
The Haber process for ammonia production demonstrates how thermochemical principles guide industrial design:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g) ΔH = -92.4 kJ/mol
This exothermic reaction requires careful temperature and pressure control to maximize yield while managing heat removal.
Pharmaceutical Development
Drug stability studies rely heavily on thermochemical analysis. Understanding how temperature affects drug decomposition helps determine storage conditions and shelf life.
Cutting-Edge Research Applications
Materials Science
Thermochemistry guides the development of new materials:
- Shape-memory alloys: Materials that “remember” their original shape when heated
- Phase-change materials: Substances that store and release large amounts of energy during melting/freezing
- Superconductors: Materials with zero electrical resistance at specific temperatures
Climate Science
Advanced climate models incorporate detailed thermochemical data to predict:
- Ocean temperature changes and thermal expansion
- Ice sheet melting rates and energy requirements
- Atmospheric chemical reactions and their energy effects
Frequently Asked Questions
Conceptual Questions
Q: Why is enthalpy change negative for exothermic reactions?
A: The sign convention reflects the system’s perspective. When a system releases energy (exothermic), it loses enthalpy, making ΔH negative. Think of it like a bank account – when you withdraw money (release energy), your balance decreases (becomes more negative).
Q: How do I know when to use q = mcΔT vs. q = CΔT?
A: Use q = mcΔT when you know the mass and specific heat of the substance. Use q = CΔT when you know the total heat capacity of the entire system (like a calorimeter constant). The key difference is that C already includes the mass factor.
Q: What’s the difference between ΔH and ΔE?
A: ΔH (enthalpy change) applies to constant-pressure processes, while ΔE (internal energy change) applies to constant-volume processes. They’re related by: ΔH = ΔE + ΔnRT, where Δn is the change in moles of gas.
Calculation Questions
Q: Why do my Hess’s Law answers sometimes differ from literature values?
A: Small differences usually arise from:
- Rounding errors in intermediate steps
- Different standard conditions (temperature, pressure)
- Experimental uncertainties in the original measurements
- Different physical states of compounds
Q: How do I handle phase changes in thermochemical calculations?
A: Remember that phase changes occur at constant temperature but require energy (fusion, vaporization). Calculate sensible heat (temperature changes) and latent heat (phase changes) separately, then add them together.
Exam Strategy Questions
Q: Should I memorize all the standard formation enthalpies?
A: No! The AP exam provides necessary thermodynamic data. Focus on understanding how to use the values rather than memorizing them. However, knowing a few common values (like water formation: -285.8 kJ/mol) can help you check if your answers are reasonable.
Q: What if I can’t remember the exact formula during the exam?
A: Focus on understanding the underlying principles. For example, if you forget the exact calorimetry formula, remember that heat gained by the solution equals heat lost by the reaction, and heat is proportional to mass, specific heat, and temperature change.
Advanced Questions
Q: How does molecular structure affect enthalpy changes?
A: Bond strength and stability play crucial roles:
- Stronger bonds require more energy to break
- More stable products release more energy when formed
- Resonance and aromaticity generally increase stability
- Larger molecules typically have higher combustion enthalpies
Q: Why don’t entropy and Gibbs free energy appear much in Unit 6?
A: Unit 6 focuses primarily on enthalpy because it’s the most straightforward energy term to measure experimentally. Entropy and Gibbs free energy are covered more thoroughly in Unit 9 (Thermodynamics), building on the foundation established here.
Conclusion & Next Steps
Your Thermochemistry Journey: From Confusion to Mastery
Congratulations! You’ve just completed a comprehensive journey through AP Chemistry Unit 6: Thermochemistry. From understanding the fundamental difference between heat and temperature to mastering complex Hess’s Law calculations, you’ve built a solid foundation in one of chemistry’s most practical and important areas.
Key Takeaways for Exam Success
As you prepare for your AP Chemistry exam, remember these crucial points:
- Thermochemistry is fundamentally about energy accounting – every joule must be accounted for
- Sign conventions matter – negative ΔH means energy leaves the system (exothermic)
- Calorimetry connects theory to experiment – understand both the concepts and the math
- Hess’s Law is your problem-solving superpower – use it to find enthalpy changes for any reaction
- Units and significant figures count – they often determine partial credit on the AP exam
Real-World Relevance
The principles you’ve learned extend far beyond the classroom. Every time you:
- Feel warmth from a campfire (exothermic combustion)
- Use a hot pack or cold pack (enthalpy of solution)
- Cook food (thermochemical reactions)
- Drive a car (combustion engine thermochemistry)
- Charge your phone (electrochemical thermodynamics)
You’re witnessing thermochemistry in action. This knowledge gives you a deeper understanding of the energy processes that power our modern world.
Next Steps in Your AP Chemistry Journey
Immediate Actions (This Week):
- Complete the 25 practice problems in this guide
- Take a timed practice test on thermochemistry
- Review and strengthen any weak areas identified
- Form a study group to discuss challenging concepts
Short-term Goals (Next Month):
- Connect thermochemistry concepts to Unit 9 (Thermodynamics)
- Practice mixed-unit problems that combine thermochemistry with other topics
- Review laboratory procedures and data analysis techniques
- Complete additional practice tests from reputable sources
Long-term Preparation (Until Exam Day):
- Maintain regular review of thermochemistry concepts
- Focus on application problems rather than rote memorization
- Practice explaining concepts to others (teaching is the best learning)
- Stay confident and trust your preparation
Advanced Study Resources
For students ready to go beyond the AP curriculum:
Recommended Reading:
- “Physical Chemistry: A Molecular Approach” by McQuarrie & Simon
- “Thermodynamics: An Engineering Approach” by Çengel & Boles
- Research papers on environmental thermochemistry
Online Resources:
- Khan Academy’s Physical Chemistry series
- MIT OpenCourseWare thermodynamics lectures
- NIST Chemistry WebBook for thermodynamic data
Laboratory Extensions:
- Design your own calorimetry experiments
- Investigate the thermochemistry of food items
- Explore phase change materials and their applications
A Personal Message
Remember, every expert was once a beginner. The struggles you face with thermochemistry today are the same challenges that have confronted countless successful chemists, engineers, and scientists. What matters is not how quickly you understand every concept, but how persistently you work to master them.
Thermochemistry might seem abstract now, but it’s the foundation for understanding everything from climate change to renewable energy, from biochemical processes to advanced materials. The time you invest in truly understanding these concepts will pay dividends throughout your scientific career.
Final Exam Strategy Reminder
As you approach your AP Chemistry exam:
- Trust your preparation – you’ve put in the work
- Stay calm under pressure – use deep breathing and positive self-talk
- Read questions carefully – many mistakes come from misreading
- Show your work clearly – partial credit can make the difference
- Manage your time wisely – don’t get stuck on any single problem
- Review your answers – catch simple calculation errors
Looking Beyond AP Chemistry
Whether you’re planning to major in chemistry, engineering, medicine, or any other science field, the analytical thinking and problem-solving skills you’ve developed through thermochemistry will serve you well. You’ve learned to:
- Break complex problems into manageable steps
- Apply mathematical relationships to physical phenomena
- Think critically about experimental design and data interpretation
- Connect molecular-level processes to macroscopic observations
These skills are transferable to virtually any STEM field and many areas beyond science.
Your Thermochemistry Toolkit
You now have a comprehensive toolkit for tackling any thermochemistry problem:
Conceptual Tools:
- Deep understanding of energy conservation
- Clear mental models of molecular processes
- Ability to visualize energy changes through diagrams
Mathematical Tools:
- Calorimetry calculations (q = mcΔT, q = CΔT)
- Hess’s Law applications
- Formation enthalpy calculations
Problem-Solving Tools:
- The IDEAL method for systematic problem solving
- Recognition of common problem types and solution strategies
- Error-checking techniques and reasonableness tests
Exam Tools:
- Time management strategies
- Common mistake awareness
- Confidence-building techniques
The Bigger Picture
Thermochemistry is more than just another unit in AP Chemistry – it’s a window into understanding how energy shapes our universe. From the nuclear fusion reactions that power stars to the biochemical processes that sustain life, thermochemical principles govern the fundamental processes of nature.
As you continue your scientific journey, carry with you the curiosity and analytical mindset that thermochemistry has helped you develop. Ask questions about the energy changes around you. Wonder about the thermochemistry of everyday processes. Most importantly, never stop learning and exploring.
Your AP Chemistry exam is just one milestone in a lifelong journey of scientific discovery. The foundation you’ve built in thermochemistry will support not just your immediate exam success, but your long-term growth as a scientifically literate citizen of the world.
Good luck on your AP Chemistry exam – you’re ready to succeed!
Remember: This guide is your reference companion, but your curiosity and dedication are your greatest assets. Keep questioning, keep learning, and keep pushing the boundaries of your understanding. The world needs scientists who can think thermochemically about the energy challenges facing our planet.
Image Credits and References
- Energy Diagrams: Expii
- Bomb Calorimeter: Britannica
- Hess’s Law Diagrams: Chemistry Steps
All thermodynamic data referenced from NIST Chemistry WebBook and standard AP Chemistry reference materials.
Read More –

1 thought on “Master AP Chemistry Unit 6: Thermochemistry – Complete Study Guide 2025”