Why Unit 9 Can Make or Break Your AP Chemistry Score
Are you struggling to connect the dots between energy changes and chemical reactions? Does the relationship between thermodynamics and electrochemistry feel like a mystery? You’re not alone. AP Chemistry Unit 9: Thermodynamics & Electrochemistry is where many students hit a wall, but it’s also where the biggest breakthroughs happen.
This unit represents 7-9% of your AP Chemistry exam, but its impact goes far beyond that percentage. The concepts you’ll master here – from predicting reaction spontaneity to understanding how batteries work – form the foundation for advanced chemistry and real-world applications.
The Challenge: Students often struggle because Unit 9 requires you to think like a chemist, combining mathematical calculations with conceptual understanding while connecting energy changes to electron flow.
The Solution: This comprehensive guide will transform these challenging concepts into your strongest assets, providing you with the tools, strategies, and confidence needed to excel on exam day.
At a Glance: Essential Formulas at a Glance
| Concept | Formula | Units | When to Use |
|---|---|---|---|
| Gibbs Free Energy | ΔG = ΔH – TΔS | J/mol or kJ/mol | Predicting spontaneity |
| Standard Free Energy | ΔG° = -RT ln K | J/mol or kJ/mol | Relating to equilibrium |
| Cell Potential | E°cell = E°cathode – E°anode | V (volts) | Galvanic cells |
| Free Energy & Cell Potential | ΔG° = -nFE° | J/mol | Connecting thermo to electro |
| Nernst Equation | E = E° – (RT/nF)ln Q | V (volts) | Non-standard conditions |
Key Constants You’ll Need
- R (Gas Constant): 8.314 J/(mol·K)
- F (Faraday Constant): 96,485 C/mol
- Standard Temperature: 298 K (25°C)
Spontaneity Quick Check
- ΔG < 0: Spontaneous (thermodynamically favored)
- ΔG > 0: Non-spontaneous (not thermodynamically favored)
- ΔG = 0: At equilibrium
Building Your Thermodynamic Toolkit
Understanding Entropy (S): The Universe’s Drive Toward Disorder
Entropy is nature’s tendency toward randomness and disorder. Think of it as the universe’s preference for chaos over order.
Key Insights:
- Entropy increases when:
- Gases are produced from liquids or solids
- More particles are created (2 → 3 particles)
- Temperature increases
- Solutions are formed from pure substances
Real-World Connection: When you drop an ice cube into hot coffee, the system naturally moves toward thermal equilibrium (maximum entropy), with the ice melting and temperatures equalizing.
Enthalpy (H) vs. Entropy (S): The Eternal Competition
Every chemical reaction involves a tug-of-war between enthalpy and entropy:
- Enthalpy-driven reactions: Very exothermic reactions (large negative ΔH) can overcome entropy decreases
- Entropy-driven reactions: Large entropy increases can drive endothermic reactions
Student Tip: Remember “HELPS” – Heat Enthalpically Lowers Product Stability when entropy opposes the reaction.
Gibbs Free Energy: The Ultimate Decision Maker
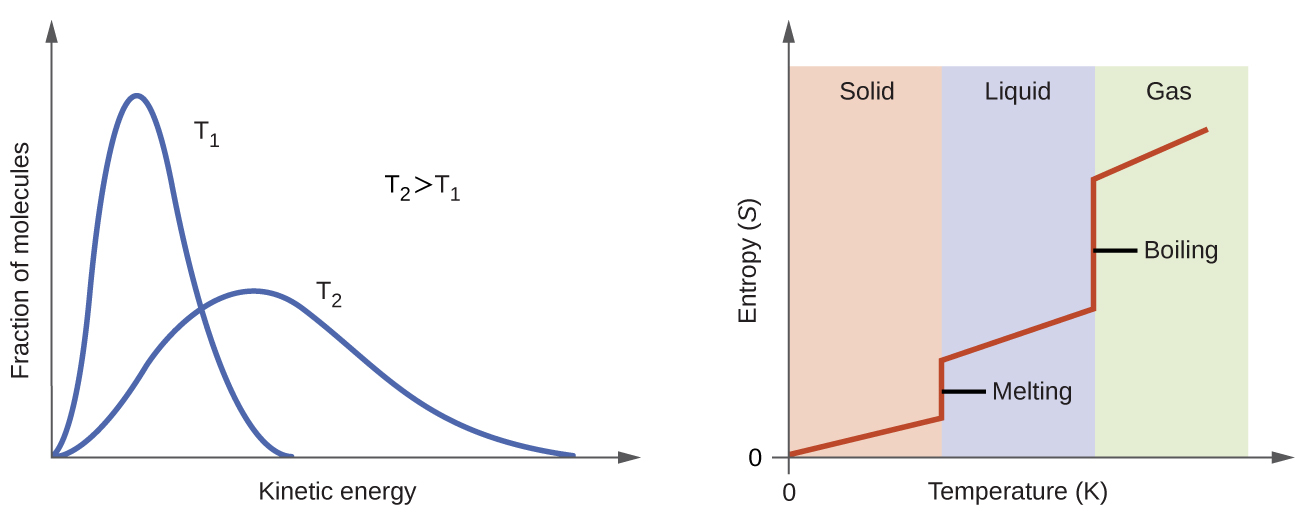
Gibbs free energy (G) is the thermodynamic quantity that determines whether a reaction will occur spontaneously. It beautifully combines both enthalpy and entropy effects:
ΔG = ΔH – TΔS
The Four Scenarios:
- ΔH < 0, ΔS > 0: Always spontaneous (exothermic + entropy increase)
- ΔH > 0, ΔS < 0: Never spontaneous (endothermic + entropy decrease)
- ΔH < 0, ΔS < 0: Spontaneous at low temperatures
- ΔH > 0, ΔS > 0: Spontaneous at high temperatures
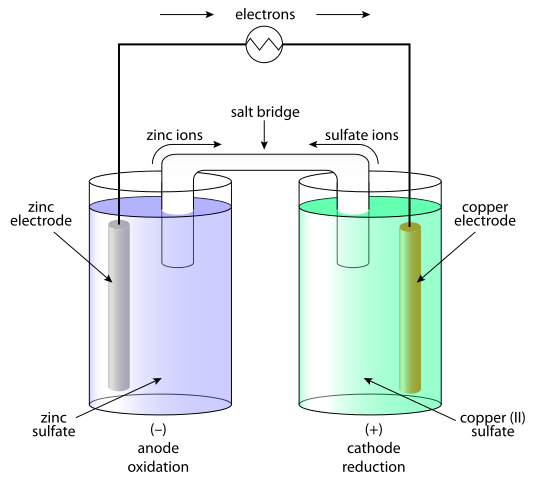
Electrochemistry: Where Chemistry Meets Electricity
Galvanic Cells: Nature’s Batteries
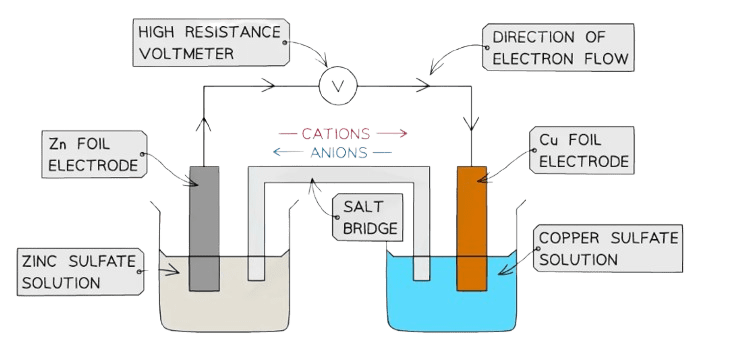
Galvanic cells convert chemical energy into electrical energy through spontaneous redox reactions.
Essential Components:
- Anode: Where oxidation occurs (electrons lost) – negative electrode
- Cathode: Where reduction occurs (electrons gained) – positive electrode
- Salt Bridge: Maintains electrical neutrality
- External Circuit: Path for electron flow
Memory Device: “An Ox, Red Cat” – Anode Oxidation, Reduction Cathode
Standard Reduction Potentials: The Electrochemical Hierarchy
Standard reduction potentials (E°) tell us which species are better oxidizing or reducing agents:
- Higher E°: Better oxidizing agent (more likely to be reduced)
- Lower E°: Better reducing agent (more likely to be oxidized)
Calculating Cell Potential:
E°cell = E°cathode – E°anode
For spontaneous reactions: E°cell > 0
Electrolytic Cells: Forcing Non-Spontaneous Reactions
Unlike galvanic cells, electrolytic cells use electrical energy to drive non-spontaneous reactions:
- External power source required
- E°cell < 0 for the desired reaction
- Applications: Electroplating, metal purification, battery charging
The Thermodynamics-Electrochemistry Connection
The Golden Equation: ΔG° = -nFE°
This equation bridges thermodynamics and electrochemistry, connecting free energy changes to cell potentials:
- n: Number of electrons transferred
- F: Faraday constant (96,485 C/mol)
- E°: Standard cell potential
Powerful Implications:
- If E°cell > 0, then ΔG° < 0 (spontaneous)
- If E°cell < 0, then ΔG° > 0 (non-spontaneous)
Equilibrium and Cell Potential
At equilibrium, both ΔG = 0 and Ecell = 0, leading to the relationship:
ΔG° = -RT ln K = -nFE°
This connects:
- Thermodynamic favorability (ΔG°)
- Equilibrium position (K)
- Electrochemical driving force (E°)
20 Multiple Choice Practice Questions
Questions 1-5: Thermodynamics Fundamentals
- Which of the following processes has the largest positive ΔS?
a) H₂O(l) → H₂O(s)
b) 2SO₂(g) + O₂(g) → 2SO₃(g)
c) NH₄Cl(s) → NH₃(g) + HCl(g)
d) 2H₂(g) + O₂(g) → 2H₂O(l)
Answer: C
Explanation: This reaction produces two gas molecules from one solid, representing the largest increase in disorder (entropy).
- For a reaction where ΔH = -85 kJ/mol and ΔS = -170 J/(mol·K), at what temperature does the reaction become non-spontaneous?
a) 298 K
b) 400 K
c) 500 K
d) 600 K
Answer: C
Explanation: At the transition point, ΔG = 0, so T = ΔH/ΔS = 85,000/170 = 500 K
- Which statement about Gibbs free energy is correct?
a) ΔG > 0 indicates a spontaneous process
b) ΔG = 0 only at absolute zero
c) ΔG° relates to standard conditions only
d) ΔG can predict reaction rate
Answer: C
Explanation: ΔG° is specifically for standard conditions (1 M, 1 atm, 25°C), while ΔG applies to any conditions.
Questions 4-8: Electrochemistry Basics
- In a galvanic cell, electrons flow:
a) From cathode to anode through the external circuit
b) From anode to cathode through the external circuit
c) Through the salt bridge
d) From positive to negative electrode
Answer: B
Explanation: Electrons always flow from the anode (where oxidation occurs) to the cathode through the external circuit.
- Given: Cu²⁺ + 2e⁻ → Cu E° = +0.34 V and Zn²⁺ + 2e⁻ → Zn E° = -0.76 V
What is E°cell for a Cu-Zn galvanic cell?
a) -1.10 V
b) -0.42 V
c) +0.42 V
d) +1.10 V
Answer: D
Explanation: E°cell = E°cathode – E°anode = 0.34 – (-0.76) = 1.10 V
Questions 9-15: Advanced Applications
- Using ΔG° = -nFE°, calculate ΔG° for the reaction in question 5:
a) -106 kJ/mol
b) -212 kJ/mol
c) +106 kJ/mol
d) +212 kJ/mol
Answer: B
Explanation: ΔG° = -nFE° = -(2)(96,485)(1.10) = -212,267 J/mol = -212 kJ/mol
- Which factor does NOT affect the cell potential of a galvanic cell?
a) Temperature
b) Concentration of reactants
c) Size of electrodes
d) Nature of the electrolyte
Answer: C
Explanation: Cell potential is intensive – it doesn’t depend on the amount of material, including electrode size.
Questions 16-20: Integrated Concepts
- For the reaction A + B → C + D, if E°cell = -0.25 V, which is true?
a) The reaction is spontaneous under standard conditions
b) ΔG° < 0 c) K > 1
d) The reaction requires external energy
Answer: D
Explanation: Negative E°cell means positive ΔG°, indicating a non-spontaneous reaction requiring external energy.
5 Free Response Problems with Solutions
Problem 1: Thermodynamic Analysis
Consider the reaction: 2NO(g) + O₂(g) → 2NO₂(g)
Given data at 298 K:
- ΔH° = -114 kJ/mol
- ΔS° = -146 J/(mol·K)
a) Calculate ΔG° at 298 K
b) Determine if the reaction is spontaneous at standard conditions
c) At what temperature would ΔG = 0?
Solution:
a) ΔG° = ΔH° – TΔS°
ΔG° = -114,000 J/mol – (298 K)(-146 J/(mol·K))
ΔG° = -114,000 + 43,508 = -70,492 J/mol = -70.5 kJ/mol
b) Since ΔG° < 0, the reaction is spontaneous at standard conditions.
c) At equilibrium, ΔG = 0:
0 = ΔH° – TΔS°
T = ΔH°/ΔS° = -114,000/-146 = 781 K
Problem 2: Galvanic Cell Analysis
Design a galvanic cell using the following half-reactions:
- Ag⁺ + e⁻ → Ag E° = +0.80 V
- Fe³⁺ + 3e⁻ → Fe E° = -0.04 V
a) Identify the anode and cathode
b) Write the overall cell reaction
c) Calculate E°cell and ΔG°
Solution:
a) Cathode: Ag⁺ + e⁻ → Ag (higher E°, reduction occurs)
Anode: Fe → Fe³⁺ + 3e⁻ (lower E°, oxidation occurs)
b) To balance electrons:
3Ag⁺ + 3e⁻ → 3Ag
Fe → Fe³⁺ + 3e⁻
Overall: 3Ag⁺ + Fe → 3Ag + Fe³⁺
c) E°cell = 0.80 – (-0.04) = 0.84 V
ΔG° = -nFE° = -(3)(96,485)(0.84) = -243,140 J/mol = -243 kJ/mol
Study Strategies and Exam Preparation
Time Management on Exam Day
Multiple Choice Strategy (90 minutes, 60 questions):
- 1.5 minutes per question average
- Unit 9 questions (4-6 expected): Allocate 2 minutes each
- Quick elimination: Cross out obviously wrong answers first
- Flag difficult questions: Return if time permits
Free Response Strategy (105 minutes, 7 questions):
- Long questions (1-3): 23 minutes each
- Short questions (4-7): 9 minutes each
- Unit 9 typically appears: 1 long question or 2 short questions
Memory Techniques for Success
For Thermodynamic Signs:
- “SHEEP” – Spontaneous Has Energy Exiting Products (ΔG < 0)
- “ICE” – In Cold Endothermic reactions need entropy increase
For Electrochemistry:
- “LEO GER” – Lose Electrons Oxidation, Gain Electrons Reduction
- “Red Cat An Ox” – Reduction Cathode, Anode Oxidation
Common Errors to Avoid
- Sign confusion in ΔG calculations
- Remember: ΔG = ΔH – TΔS (subtraction, not addition)
- Electrode identification mistakes
- Higher E° = cathode (reduction)
- Lower E° = anode (oxidation)
- Unit conversion errors
- Always convert J to kJ or vice versa consistently
- Convert Celsius to Kelvin: K = °C + 273
- Equilibrium vs. standard conditions
- ΔG° is for standard conditions
- ΔG = 0 only at equilibrium
Real-World Applications and Connections
Battery Technology: From Theory to Practice
Lithium-Ion Batteries:
The principles you’re learning directly apply to modern battery technology:
- Anode: Lithium metal (oxidation: Li → Li⁺ + e⁻)
- Cathode: Metal oxide (reduction of metal ions)
- Cell potential: ~3.7 V per cell
Why This Matters:
- Electric vehicle development
- Smartphone battery life
- Renewable energy storage
Industrial Electroplating
Electrolytic cells are used extensively in manufacturing:
- Chrome plating: Aesthetic and corrosion protection
- Gold plating: Electronics and jewelry
- Copper purification: 99.99% pure copper production
Biological Systems
Cellular Respiration:
Your body uses thermodynamic principles:
- ΔG° for glucose oxidation: -2870 kJ/mol
- ATP synthesis: Couples unfavorable reactions with favorable ones
- Electron transport chain: Similar to electrochemical cells
Advanced Applications for Top Scorers
The Nernst Equation: Beyond Standard Conditions
For non-standard conditions:
E = E° – (RT/nF)ln Q
When concentrations change:
- Q > 1: Cell potential decreases
- Q < 1: Cell potential increases
- Q = K: Cell potential = 0 (equilibrium)
Concentration Cells
Special galvanic cells where:
- Same elements in both half-cells
- Different concentrations create potential
- E°cell = 0, but Ecell ≠ 0 due to concentration differences
Corrosion: Unwanted Electrochemistry
Iron rusting:
- Anode reaction: Fe → Fe²⁺ + 2e⁻
- Cathode reaction: O₂ + 4H⁺ + 4e⁻ → 2H₂O
- Prevention: Galvanization (zinc coating acts as sacrificial anode)
FAQs
Q1: How do I know when to use ΔG vs. ΔG°?
Answer: Use ΔG° when dealing with standard conditions (1 M concentrations, 1 atm pressure, 25°C). Use ΔG for non-standard conditions or when determining equilibrium (ΔG = 0).
Q2: Why does the salt bridge not affect cell potential?
Answer: The salt bridge maintains electrical neutrality but doesn’t participate in the redox reaction. It allows ion flow to complete the circuit without affecting the electron transfer that determines cell potential.
Q3: Can an endothermic reaction be spontaneous?
Answer: Yes! If ΔS is sufficiently positive, the TΔS term can make ΔG negative even when ΔH is positive. Example: Ice melting above 0°C.
Q4: What’s the difference between galvanic and electrolytic cells?
Answer:
- Galvanic: Spontaneous reactions, produce electricity, E°cell > 0
- Electrolytic: Non-spontaneous reactions, consume electricity, E°cell < 0
Q5: How do I approach thermodynamics problems systematically?
Answer: Follow the “GIST” method:
- Given data identification
- Identify what you’re solving for
- Select appropriate equations
- Track units and significant figures
Q6: What if I forget the Faraday constant during the exam?
Answer: The AP Chemistry exam provides a formula sheet with F = 96,485 C/mol. Focus on understanding relationships rather than memorizing constants.
Q7: How important is understanding vs. memorization for Unit 9?
Answer: Understanding is crucial. The exam tests conceptual connections between thermodynamics and electrochemistry. Memorizing formulas without understanding leads to errors in complex problems.
Conclusion and Next Steps
What You’ve Mastered
Congratulations! You’ve now built a comprehensive understanding of AP Chemistry Unit 9: Thermodynamics & Electrochemistry. You can:
✅ Predict reaction spontaneity using Gibbs free energy
✅ Analyze electrochemical cells and calculate cell potentials
✅ Connect thermodynamic and electrochemical concepts through ΔG° = -nFE°
✅ Solve complex problems involving entropy, enthalpy, and free energy
✅ Apply concepts to real-world situations from batteries to biological systems
Your Exam Day Action Plan
Week Before the Exam:
- Review this guide – Focus on weak areas identified through practice
- Complete timed practice – Use official College Board materials
- Form study groups – Explain concepts to others to reinforce learning
- Rest and nutrition – Your brain needs fuel and recovery
Day of the Exam:
- Arrive early and calm – Bring required materials and confidence
- Read questions carefully – Identify what’s being asked before calculating
- Show your work – Partial credit is awarded on free response questions
- Manage your time – Don’t get stuck on one difficult problem
Beyond the AP Exam
The concepts you’ve mastered extend far beyond test day:
Academic Pathways:
- Physical Chemistry: Advanced thermodynamics and kinetics
- Materials Science: Battery and fuel cell development
- Biochemistry: Energy metabolism and enzyme function
- Chemical Engineering: Process optimization and energy efficiency
Career Applications:
- Battery Technology: Electric vehicles and energy storage
- Environmental Science: Green chemistry and sustainability
- Medicine: Drug development and biological energy systems
- Manufacturing: Electroplating and metal processing
Resources for Continued Success
Official College Board Resources:
Additional Practice:
- Khan Academy AP Chemistry
- Albert.io Practice Problems
- Barron’s AP Chemistry Review Book
Final Motivation
Remember, every AP Chemistry success story started with a student just like you, facing the same challenges and uncertainties. The difference between good and great performance lies not in natural ability, but in persistent effort and strategic preparation.
You’ve invested in comprehensive preparation through this guide. Trust your preparation, apply your knowledge confidently, and remember that mastering thermodynamics and electrochemistry opens doors to understanding some of the most important processes in chemistry and life itself.
Your success in Unit 9 will not only boost your AP Chemistry score but also provide you with powerful tools for understanding the energy changes that drive our world.
Good luck, and remember – you’ve got this!
Read More –

